Internal Kinetic Energy Formula . For an ideal monoatomic gas, this is just the translational kinetic energy of. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. The symbol u is used for the internal energy and the unit of. The heat flow is equal to the change in the internal energy of the system plus the pv work. The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. The change in the internal energy of a system is the sum of the heat transferred and the work done. Internal energy involves energy on the microscopic scale. If an object is rotating, it could have rotational kinetic energy. If it is vibrating, it could have vibrational kinetic energy.
from socratic.org
If an object is rotating, it could have rotational kinetic energy. The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. Internal energy involves energy on the microscopic scale. If it is vibrating, it could have vibrational kinetic energy. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. The heat flow is equal to the change in the internal energy of the system plus the pv work. The change in the internal energy of a system is the sum of the heat transferred and the work done. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. For an ideal monoatomic gas, this is just the translational kinetic energy of. The symbol u is used for the internal energy and the unit of.
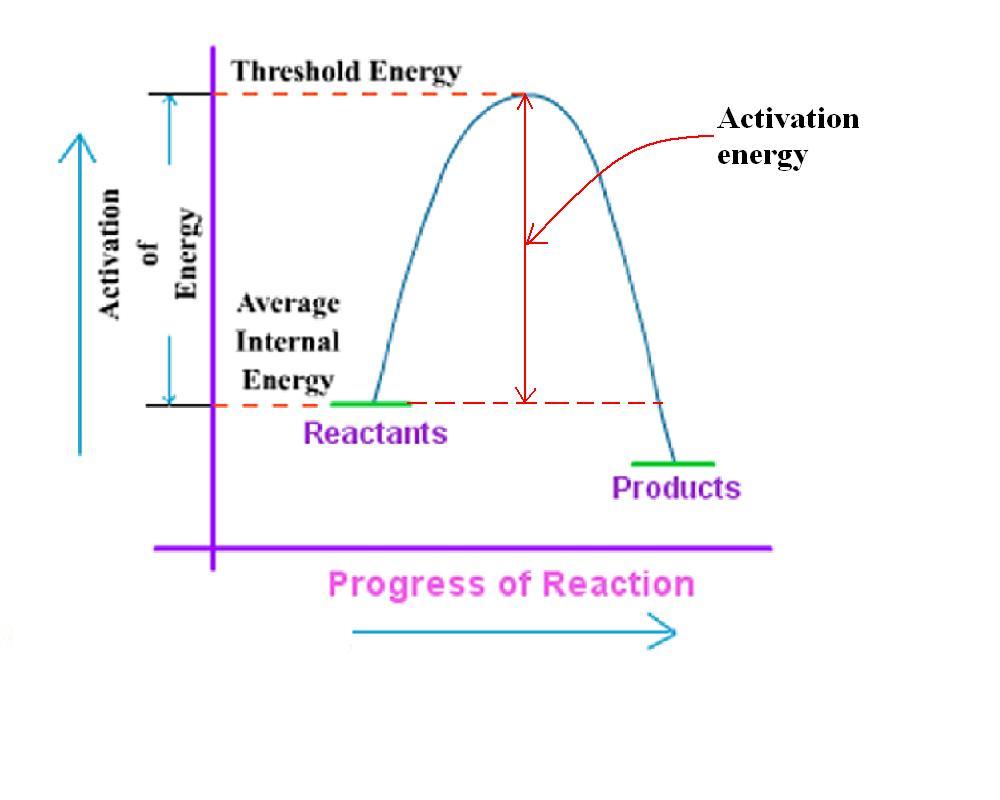
What is activation energy? What is threshold energy? What are the
Internal Kinetic Energy Formula For an ideal monoatomic gas, this is just the translational kinetic energy of. For an ideal monoatomic gas, this is just the translational kinetic energy of. If it is vibrating, it could have vibrational kinetic energy. Internal energy involves energy on the microscopic scale. The change in the internal energy of a system is the sum of the heat transferred and the work done. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. The heat flow is equal to the change in the internal energy of the system plus the pv work. The symbol u is used for the internal energy and the unit of. If an object is rotating, it could have rotational kinetic energy.
From www.researchgate.net
Histories of energy, internal energy, and their ratio of the Internal Kinetic Energy Formula If it is vibrating, it could have vibrational kinetic energy. The symbol u is used for the internal energy and the unit of. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. The heat flow is equal to the change in the internal energy of the system. Internal Kinetic Energy Formula.
From www.youtube.com
What Is Energy, Formula Of EnergyAayuPhysics YouTube Internal Kinetic Energy Formula If an object is rotating, it could have rotational kinetic energy. Internal energy involves energy on the microscopic scale. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. If it is vibrating, it could have vibrational kinetic energy. Internal energy is a measure of the total energy of. Internal Kinetic Energy Formula.
From www.careerpower.in
Energy Definition, Example and Derivation Internal Kinetic Energy Formula For an ideal monoatomic gas, this is just the translational kinetic energy of. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. The change in the internal energy of a system is the sum of the heat transferred and the work done. If an object is rotating, it. Internal Kinetic Energy Formula.
From haipernews.com
How To Calculate Energy And Potential Energy Haiper Internal Kinetic Energy Formula The symbol u is used for the internal energy and the unit of. The change in the internal energy of a system is the sum of the heat transferred and the work done. The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. Internal energy. Internal Kinetic Energy Formula.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID6710377 Internal Kinetic Energy Formula Internal energy involves energy on the microscopic scale. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. The heat flow is equal to the change in the internal energy of the system plus the pv work. If it is vibrating, it could have vibrational kinetic energy. For. Internal Kinetic Energy Formula.
From ar.inspiredpencil.com
Energy Temperature Internal Kinetic Energy Formula For an ideal monoatomic gas, this is just the translational kinetic energy of. The symbol u is used for the internal energy and the unit of. Internal energy involves energy on the microscopic scale. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. The heat flow is equal. Internal Kinetic Energy Formula.
From online-learning-college.com
Gravitational potential and energy Calculating energies Internal Kinetic Energy Formula The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. If it is vibrating, it could have vibrational kinetic energy. If an object is. Internal Kinetic Energy Formula.
From socratic.org
Question d21dd Socratic Internal Kinetic Energy Formula If it is vibrating, it could have vibrational kinetic energy. For an ideal monoatomic gas, this is just the translational kinetic energy of. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. If an object is rotating, it could have rotational kinetic energy. The heat flow is equal. Internal Kinetic Energy Formula.
From www.mechanicaleducation.com
Difference Between Energy And Potential Energy Mechanical Internal Kinetic Energy Formula If an object is rotating, it could have rotational kinetic energy. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. Internal energy involves. Internal Kinetic Energy Formula.
From www.vrogue.co
Energy Definition Formula Examples Teachoo vrogue.co Internal Kinetic Energy Formula The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. For an ideal monoatomic gas, this is just the translational kinetic energy of. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and.. Internal Kinetic Energy Formula.
From ppt-online.org
theory of ideal gases презентация онлайн Internal Kinetic Energy Formula The heat flow is equal to the change in the internal energy of the system plus the pv work. The change in the internal energy of a system is the sum of the heat transferred and the work done. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic. Internal Kinetic Energy Formula.
From www.youtube.com
8.18 What is the internal energy of an ideal gas? YouTube Internal Kinetic Energy Formula If an object is rotating, it could have rotational kinetic energy. The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. For an ideal monoatomic gas, this is just the translational kinetic energy of. The change in the internal energy of a system is the. Internal Kinetic Energy Formula.
From waiferx.blogspot.com
Pdog's blog boring but important Physics presentation internal Internal Kinetic Energy Formula Internal energy involves energy on the microscopic scale. For an ideal monoatomic gas, this is just the translational kinetic energy of. If it is vibrating, it could have vibrational kinetic energy. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. Internal energy is a measure of the total. Internal Kinetic Energy Formula.
From www.slideserve.com
PPT C H A P T E R 14 The Ideal Gas Law and Theory PowerPoint Internal Kinetic Energy Formula The symbol u is used for the internal energy and the unit of. If it is vibrating, it could have vibrational kinetic energy. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. The heat flow is equal to the change in the internal energy of the system. Internal Kinetic Energy Formula.
From www.tec-science.com
Internal energy & first law of thermodynamics tecscience Internal Kinetic Energy Formula Internal energy involves energy on the microscopic scale. The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. The heat flow is equal to the change in the internal energy of the system plus the pv work. Internal energy is a measure of the total. Internal Kinetic Energy Formula.
From jasminel-caller.blogspot.com
Formule Energie Energy and Potential Energy YouTube Internal Kinetic Energy Formula For an ideal monoatomic gas, this is just the translational kinetic energy of. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. The change in the. Internal Kinetic Energy Formula.
From www.tessshebaylo.com
First Law Of Thermodynamics Equations Tessshebaylo Internal Kinetic Energy Formula For an ideal monoatomic gas, this is just the translational kinetic energy of. The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. Internal energy involves energy on the microscopic scale. The heat flow is equal to the change in the internal energy of the. Internal Kinetic Energy Formula.
From eduinput.com
EnergyDefinition,Types,And Work Energy Principle in Term of Internal Kinetic Energy Formula The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. The heat flow is equal to the change in the internal energy of the. Internal Kinetic Energy Formula.
From www.researchgate.net
Internal energy, energy and total energy versus time Internal Kinetic Energy Formula For an ideal monoatomic gas, this is just the translational kinetic energy of. If it is vibrating, it could have vibrational kinetic energy. Internal energy involves energy on the microscopic scale. The heat flow is equal to the change in the internal energy of the system plus the pv work. Internal energy is a measure of the total energy of. Internal Kinetic Energy Formula.
From testbook.com
Internal Energy Formula Learn its Concept, Formula and Examples Internal Kinetic Energy Formula Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. The change in the internal energy of a system is the sum of the heat transferred and the work done. The symbol u is used for the internal energy and the unit of. Internal energy involves energy on. Internal Kinetic Energy Formula.
From whatsinsight.org
What is Internal Energy? RealLife Examples What's Insight Internal Kinetic Energy Formula For an ideal monoatomic gas, this is just the translational kinetic energy of. If an object is rotating, it could have rotational kinetic energy. The heat flow is equal to the change in the internal energy of the system plus the pv work. If it is vibrating, it could have vibrational kinetic energy. Internal energy is a measure of the. Internal Kinetic Energy Formula.
From www.pinterest.co.uk
Energy Equation in Joules (kgm/s^2) タイトルロゴ Internal Kinetic Energy Formula The symbol u is used for the internal energy and the unit of. The heat flow is equal to the change in the internal energy of the system plus the pv work. Internal energy involves energy on the microscopic scale. The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas),. Internal Kinetic Energy Formula.
From www.myxxgirl.com
What Is Energy Definition Examples Equation And Faqs My XXX Internal Kinetic Energy Formula The heat flow is equal to the change in the internal energy of the system plus the pv work. Internal energy involves energy on the microscopic scale. If it is vibrating, it could have vibrational kinetic energy. The symbol u is used for the internal energy and the unit of. For an ideal monoatomic gas, this is just the translational. Internal Kinetic Energy Formula.
From slideplayer.com
PHYSICS 197 Section 1 Chapter C9 Potential Energy Graphs ppt download Internal Kinetic Energy Formula Internal energy involves energy on the microscopic scale. If it is vibrating, it could have vibrational kinetic energy. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas),. Internal Kinetic Energy Formula.
From www.youtube.com
Theory Internal Energy and Molar Specific Heat. Level 2 Internal Kinetic Energy Formula Internal energy involves energy on the microscopic scale. The heat flow is equal to the change in the internal energy of the system plus the pv work. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. For an ideal monoatomic gas, this is just the translational kinetic energy. Internal Kinetic Energy Formula.
From engineerfix.com
What Is Energy? Definition, Examples, Equation, and FAQs Internal Kinetic Energy Formula Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. For an ideal monoatomic gas, this is just the translational kinetic energy of. The amount of kinetic. Internal Kinetic Energy Formula.
From mmerevise.co.uk
Thermal Energy Transfer Questions and Revision MME Internal Kinetic Energy Formula If an object is rotating, it could have rotational kinetic energy. The symbol u is used for the internal energy and the unit of. The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. Internal energy is a measure of the total energy of a. Internal Kinetic Energy Formula.
From www.numerade.com
SOLVED An object with energy K explodes into two pieces, each Internal Kinetic Energy Formula Internal energy involves energy on the microscopic scale. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. The amount of kinetic and potential energy a substance contains depends on the phases of matter (solid, liquid or gas), this is known as the. The symbol u is used. Internal Kinetic Energy Formula.
From www.youtube.com
How To Calculate The Average Translational Energy of Molecules Internal Kinetic Energy Formula The heat flow is equal to the change in the internal energy of the system plus the pv work. If an object is rotating, it could have rotational kinetic energy. An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. The symbol u is used for the internal energy. Internal Kinetic Energy Formula.
From www.slideshare.net
Physics Chapter 14 Theory of Gases Internal Kinetic Energy Formula An energy form inherent in every system is the internal energy, which arises from the molecular state of motion of matter. For an ideal monoatomic gas, this is just the translational kinetic energy of. The symbol u is used for the internal energy and the unit of. Internal energy is a measure of the total energy of a closed system. Internal Kinetic Energy Formula.
From byjus.com
The dimensional formula of energy is Internal Kinetic Energy Formula If an object is rotating, it could have rotational kinetic energy. If it is vibrating, it could have vibrational kinetic energy. Internal energy involves energy on the microscopic scale. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. The symbol u is used for the internal energy. Internal Kinetic Energy Formula.
From www.youtube.com
Theory of Gases and Internal Energy YouTube Internal Kinetic Energy Formula Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. If an object is rotating, it could have rotational kinetic energy. If it is vibrating, it could have vibrational kinetic energy. The change in the internal energy of a system is the sum of the heat transferred and. Internal Kinetic Energy Formula.
From www.chegg.com
Solved 2. The change in internal energy (KJ/kmol) of an Internal Kinetic Energy Formula For an ideal monoatomic gas, this is just the translational kinetic energy of. The heat flow is equal to the change in the internal energy of the system plus the pv work. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. Internal energy involves energy on the. Internal Kinetic Energy Formula.
From socratic.org
What is activation energy? What is threshold energy? What are the Internal Kinetic Energy Formula The symbol u is used for the internal energy and the unit of. If an object is rotating, it could have rotational kinetic energy. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. Internal energy involves energy on the microscopic scale. The change in the internal energy. Internal Kinetic Energy Formula.
From www.engineeringstream.com
LAW OF THERMODYNAMICS Engineering Stream Internal Kinetic Energy Formula The change in the internal energy of a system is the sum of the heat transferred and the work done. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and. If it is vibrating, it could have vibrational kinetic energy. The symbol u is used for the internal. Internal Kinetic Energy Formula.