Solutions And Dilutions Worksheet . When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the. M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. perform the following dilutions calculation. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. 1) for each of the following solutions, the number of moles of solute is given, followed by the. Then use that as the initial concentration for the second dilution to find the concentration of. today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock solutions), and then how one. A solution is made by adding 27.5 g of calcium fluoride to. first find the concentration of solution a. molarity and dilution worksheet name:
from www.studocu.com
M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. perform the following dilutions calculation. Then use that as the initial concentration for the second dilution to find the concentration of. A solution is made by adding 27.5 g of calcium fluoride to. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the. first find the concentration of solution a. molarity and dilution worksheet name: When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock solutions), and then how one.
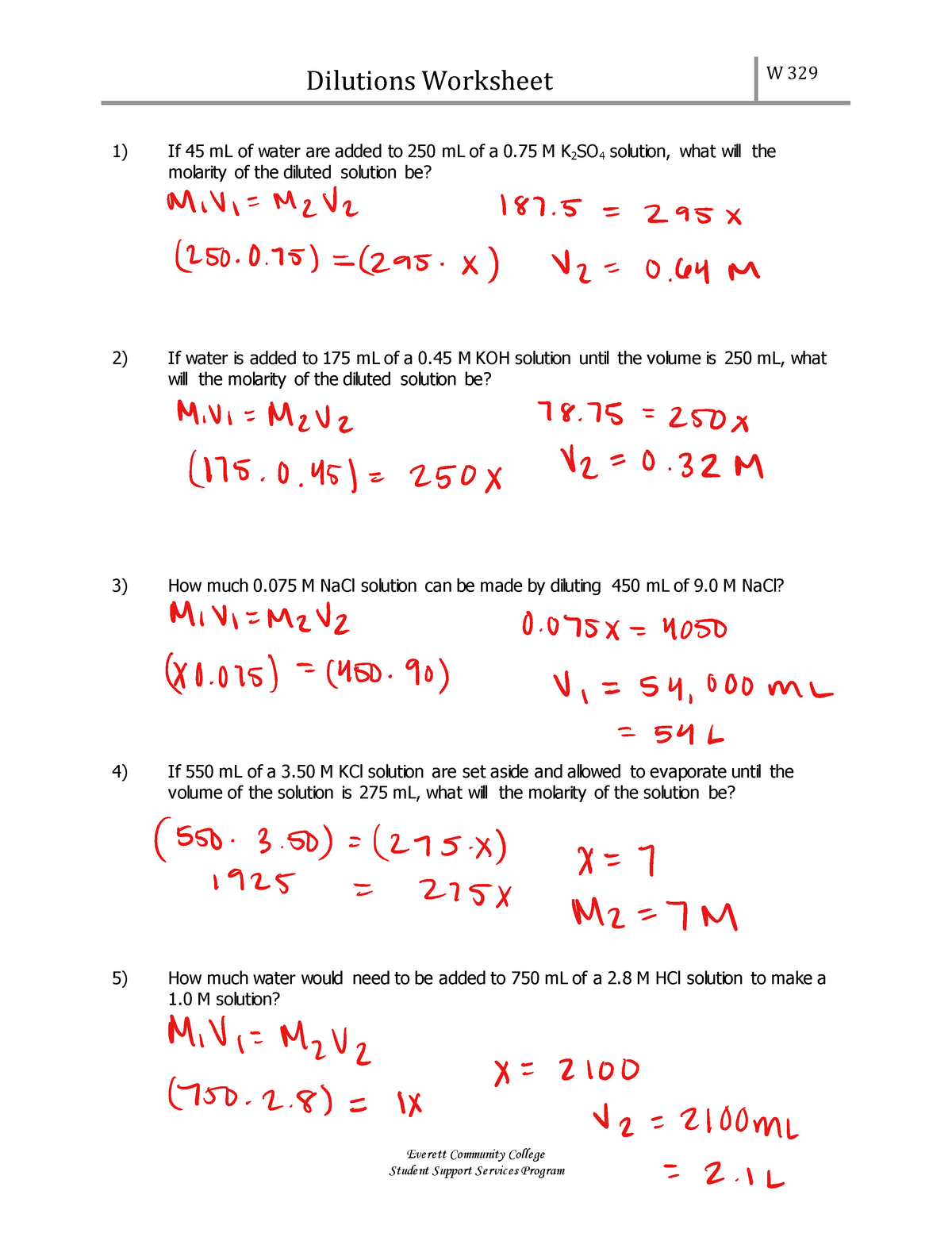
Dilution Worksheet eskandari summer Dilutions Worksheet W 329
Solutions And Dilutions Worksheet 1) for each of the following solutions, the number of moles of solute is given, followed by the. M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. A solution is made by adding 27.5 g of calcium fluoride to. perform the following dilutions calculation. 1) for each of the following solutions, the number of moles of solute is given, followed by the. When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. Then use that as the initial concentration for the second dilution to find the concentration of. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the. molarity and dilution worksheet name: today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock solutions), and then how one. first find the concentration of solution a.
From www.chegg.com
Solved Solutions and Dilutions Worksheet 1.Determine how to Solutions And Dilutions Worksheet Then use that as the initial concentration for the second dilution to find the concentration of. perform the following dilutions calculation. molarity and dilution worksheet name: When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. Add more solvent to a solution, thus volume. Solutions And Dilutions Worksheet.
From learningisidro.z13.web.core.windows.net
Making Dilutions Worksheets Solutions And Dilutions Worksheet A solution is made by adding 27.5 g of calcium fluoride to. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. molarity and dilution worksheet name: M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. perform the following dilutions calculation. today we. Solutions And Dilutions Worksheet.
From worksheets.clipart-library.com
Preparing Solutions & Dilutions worksheet Live Worksheets Solutions And Dilutions Worksheet Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the. A solution is made by adding 27.5 g of calcium fluoride to. molarity and dilution worksheet name: today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock solutions),. Solutions And Dilutions Worksheet.
From www.liveworksheets.com
Solution Prep and Dilutions worksheet Live Worksheets Solutions And Dilutions Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. A solution is made by adding 27.5 g of calcium fluoride to. 1) for each of the following solutions, the number of moles of solute is given, followed by the. Add more solvent to a solution, thus volume of solution. Solutions And Dilutions Worksheet.
From www.studocu.com
Pipetting, Solutions, and Dilutions Worksheet Pipetting, Solutions Solutions And Dilutions Worksheet Then use that as the initial concentration for the second dilution to find the concentration of. When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. first find the concentration of solution. Solutions And Dilutions Worksheet.
From worksheets.clipart-library.com
Preparing Solutions & Dilutions worksheet Live Worksheets Solutions And Dilutions Worksheet When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. perform the following dilutions calculation. first find the concentration of solution a. M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. molarity and dilution worksheet name: Then use that. Solutions And Dilutions Worksheet.
From www.studocu.com
Pipetting, Solutions, and Dilutions Worksheet Pipetting, Solutions Solutions And Dilutions Worksheet When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. first find the concentration of solution a. Add more solvent to a solution, thus volume of solution increases while moles of solute. Solutions And Dilutions Worksheet.
From www.studocu.com
Calculations solutions and dilutions Calculations Solutions and Solutions And Dilutions Worksheet When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. A solution is made by adding 27.5 g of calcium fluoride to. M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. today we are going to think more deeply about how. Solutions And Dilutions Worksheet.
From worksheets.ekocraft-appleleaf.com
Serial Dilution Worksheet Worksheets For Kindergarten Solutions And Dilutions Worksheet Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the. perform the following dilutions calculation. today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock solutions), and then how one. molarity and dilution worksheet name: M1v1 =. Solutions And Dilutions Worksheet.
From kidsworksheetfun.com
Molarity And Dilution Worksheet kidsworksheetfun Solutions And Dilutions Worksheet first find the concentration of solution a. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the. 1) for each of the following solutions, the number of moles of solute is given, followed by the. molarity and dilution worksheet name: M1v1 = m2v2 (m = molarity of solution, v=. Solutions And Dilutions Worksheet.
From www.chegg.com
Solved Solutions and Dilutions. Worksheet 3. Here are some Solutions And Dilutions Worksheet M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. molarity and dilution worksheet name: Then use that as the initial concentration for the second dilution to find the concentration of. first find the concentration of solution a. today we are going to think more deeply about how you would physically prepare solutions of. Solutions And Dilutions Worksheet.
From worksheets.clipart-library.com
Quiz & Worksheet How to Calculate Dilution of Solutions Solutions And Dilutions Worksheet first find the concentration of solution a. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the. When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. perform the following dilutions calculation. Then use that. Solutions And Dilutions Worksheet.
From www.liveworksheets.com
Preparing Standard Solution Through Dilution method worksheet Live Solutions And Dilutions Worksheet today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock solutions), and then how one. molarity and dilution worksheet name: When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. Add more solvent. Solutions And Dilutions Worksheet.
From studylib.net
Dilution Solutions And Dilutions Worksheet When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. first find the concentration of solution a. M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. molarity and dilution worksheet name: Add more solvent to a solution, thus volume of. Solutions And Dilutions Worksheet.
From www.studocu.com
Dilution Worksheet eskandari summer Dilutions Worksheet W 329 Solutions And Dilutions Worksheet M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the. perform the following dilutions calculation. today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock. Solutions And Dilutions Worksheet.
From www.liveworksheets.com
Solution Prep and Dilutions worksheet Live Worksheets Solutions And Dilutions Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. first find the concentration of solution a. today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock solutions), and then how one. perform the following dilutions. Solutions And Dilutions Worksheet.
From studylib.net
Dilutions Worksheet Solutions And Dilutions Worksheet A solution is made by adding 27.5 g of calcium fluoride to. 1) for each of the following solutions, the number of moles of solute is given, followed by the. Then use that as the initial concentration for the second dilution to find the concentration of. Add more solvent to a solution, thus volume of solution increases while moles of. Solutions And Dilutions Worksheet.
From kidsworksheetfun.com
Serial Dilutions Worksheet Answers Kidsworksheetfun Solutions And Dilutions Worksheet 1) for each of the following solutions, the number of moles of solute is given, followed by the. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. molarity and dilution worksheet name: Then use that as the initial concentration for the second dilution to find the concentration of.. Solutions And Dilutions Worksheet.
From www.youtube.com
03 The Dilution Solution Worksheet Key YouTube Solutions And Dilutions Worksheet Then use that as the initial concentration for the second dilution to find the concentration of. 1) for each of the following solutions, the number of moles of solute is given, followed by the. first find the concentration of solution a. A solution is made by adding 27.5 g of calcium fluoride to. today we are going to. Solutions And Dilutions Worksheet.
From studylib.net
Solutions & Dilutions Worksheet Solutions And Dilutions Worksheet first find the concentration of solution a. 1) for each of the following solutions, the number of moles of solute is given, followed by the. When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. Then use that as the initial concentration for the second. Solutions And Dilutions Worksheet.
From www.chegg.com
Solved Dilution Worksheet 2 Show all work in the spaces Solutions And Dilutions Worksheet A solution is made by adding 27.5 g of calcium fluoride to. Then use that as the initial concentration for the second dilution to find the concentration of. today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock solutions), and then how one. Add more solvent to a. Solutions And Dilutions Worksheet.
From thekidsworksheet.com
Solutions And Dilutions Worksheet Thekidsworksheet Solutions And Dilutions Worksheet Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the. M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock solutions), and then how one. A. Solutions And Dilutions Worksheet.
From learningschoolgraciauwb.z4.web.core.windows.net
Molarity And Dilution Worksheet Solutions And Dilutions Worksheet first find the concentration of solution a. When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. M1v1 = m2v2 (m = molarity of solution,. Solutions And Dilutions Worksheet.
From thekidsworksheet.com
Dilutions Worksheet Answers Thekidsworksheet Solutions And Dilutions Worksheet M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. perform the following dilutions calculation. A solution is made by adding 27.5 g of calcium fluoride to. When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. 1) for each of the. Solutions And Dilutions Worksheet.
From www.formsbank.com
Dilution Worksheet With Answers printable pdf download Solutions And Dilutions Worksheet 1) for each of the following solutions, the number of moles of solute is given, followed by the. today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock solutions), and then how one. first find the concentration of solution a. molarity and dilution worksheet name: 1). Solutions And Dilutions Worksheet.
From thekidsworksheet.com
Solutions And Dilutions Worksheet Thekidsworksheet Solutions And Dilutions Worksheet Then use that as the initial concentration for the second dilution to find the concentration of. A solution is made by adding 27.5 g of calcium fluoride to. When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. 1) if i add 25 ml of water. Solutions And Dilutions Worksheet.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Solutions And Dilutions Worksheet molarity and dilution worksheet name: Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the. first find the concentration of solution a. perform the following dilutions calculation. A solution is made by adding 27.5 g of calcium fluoride to. today we are going to think more deeply. Solutions And Dilutions Worksheet.
From www.formsbank.com
Dilutions And Solutions Worksheet printable pdf download Solutions And Dilutions Worksheet 1) for each of the following solutions, the number of moles of solute is given, followed by the. When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less than,. perform the following dilutions calculation. first find the concentration of solution a. molarity and dilution. Solutions And Dilutions Worksheet.
From studylib.net
Dilutions Worksheet Solutions And Dilutions Worksheet Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the. perform the following dilutions calculation. 1) for each of the following solutions, the number of moles of solute is given, followed by the. Then use that as the initial concentration for the second dilution to find the concentration of. . Solutions And Dilutions Worksheet.
From materialschoolklein.z13.web.core.windows.net
Dilutions Worksheet Chemistry Answers Solutions And Dilutions Worksheet molarity and dilution worksheet name: 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock solutions), and then how one. perform the following dilutions calculation. 1). Solutions And Dilutions Worksheet.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Solutions And Dilutions Worksheet A solution is made by adding 27.5 g of calcium fluoride to. first find the concentration of solution a. Then use that as the initial concentration for the second dilution to find the concentration of. 1) for each of the following solutions, the number of moles of solute is given, followed by the. perform the following dilutions calculation.. Solutions And Dilutions Worksheet.
From pngmusson.blogspot.com
Dilution Calculations Worksheet 2 / Png Musson Solutions And Dilutions Worksheet Then use that as the initial concentration for the second dilution to find the concentration of. first find the concentration of solution a. M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less. Solutions And Dilutions Worksheet.
From study.com
Quiz & Worksheet How to Calculate Dilution of Solutions Solutions And Dilutions Worksheet A solution is made by adding 27.5 g of calcium fluoride to. M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. perform the following dilutions calculation. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. Add more solvent to a solution, thus volume of. Solutions And Dilutions Worksheet.
From studylib.net
solutions worksheet Solutions And Dilutions Worksheet 1) for each of the following solutions, the number of moles of solute is given, followed by the. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. When a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than,. Solutions And Dilutions Worksheet.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Solutions And Dilutions Worksheet M1v1 = m2v2 (m = molarity of solution, v= volume of solution) 1. first find the concentration of solution a. today we are going to think more deeply about how you would physically prepare solutions of a specified concentration (called stock solutions), and then how one. molarity and dilution worksheet name: perform the following dilutions calculation.. Solutions And Dilutions Worksheet.