Copper Oxide And Hydrochloric Acid Colour Change . When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. When the two are mixed together, the solution turns green because the copper reacts with the acid to form. Learn about basic oxides, acid. The solution of copper(ii) chloride is. Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. Copper oxide is black in colour. At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂).
from jaxkruwfletcher.blogspot.com
Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. Copper oxide is black in colour. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. Learn about basic oxides, acid. When the two are mixed together, the solution turns green because the copper reacts with the acid to form. The solution of copper(ii) chloride is. At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂).
Copper Oxide Sulfuric Acid JaxkruwFletcher
Copper Oxide And Hydrochloric Acid Colour Change Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. Learn about basic oxides, acid. At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. The solution of copper(ii) chloride is. Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). Copper oxide is black in colour. When the two are mixed together, the solution turns green because the copper reacts with the acid to form.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Copper Oxide And Hydrochloric Acid Colour Change Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). Learn about basic oxides, acid. When the two are mixed together, the solution turns green because the copper reacts. Copper Oxide And Hydrochloric Acid Colour Change.
From askfilo.com
Recall from Chapter 2, how copper oxide reacts with hydrochloric acid. We.. Copper Oxide And Hydrochloric Acid Colour Change When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. The solution of copper(ii) chloride is. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). Copper oxide is black in colour. Learn about basic oxides, acid. At a guess, you're seeing the copper turn pink because. Copper Oxide And Hydrochloric Acid Colour Change.
From www.numerade.com
SOLVED Write balanced chemical equations for each reaction and Copper Oxide And Hydrochloric Acid Colour Change Learn about basic oxides, acid. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). Copper oxide is black in colour. Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity.. Copper Oxide And Hydrochloric Acid Colour Change.
From www.numerade.com
SOLVED aqueous barium hydroxide and sulfuric acid aqueous barium Copper Oxide And Hydrochloric Acid Colour Change Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. When the two are mixed together, the solution turns green because the copper reacts with the acid to form. The solution of copper(ii) chloride is. At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting. Copper Oxide And Hydrochloric Acid Colour Change.
From www.nagwa.com
Question Video Identifying the Products When a Metal Oxide Reacts with Copper Oxide And Hydrochloric Acid Colour Change The solution of copper(ii) chloride is. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). When the two are mixed together, the solution turns green because. Copper Oxide And Hydrochloric Acid Colour Change.
From www.youtube.com
Copper & Hydrochloric Acid Ligand Substitution YouTube Copper Oxide And Hydrochloric Acid Colour Change Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. Copper oxide is black in colour. At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii). Copper Oxide And Hydrochloric Acid Colour Change.
From openoregon.pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry Copper Oxide And Hydrochloric Acid Colour Change The solution of copper(ii) chloride is. Learn about basic oxides, acid. Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. When the two are mixed together, the solution turns green because the copper reacts with the acid to form. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride. Copper Oxide And Hydrochloric Acid Colour Change.
From www.toppr.com
Take a small amount of copper oxide in a beaker and add dilute Copper Oxide And Hydrochloric Acid Colour Change When the two are mixed together, the solution turns green because the copper reacts with the acid to form. Copper oxide is black in colour. The solution of copper(ii) chloride is. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. At a guess, you're seeing the copper turn pink because it. Copper Oxide And Hydrochloric Acid Colour Change.
From brainly.com
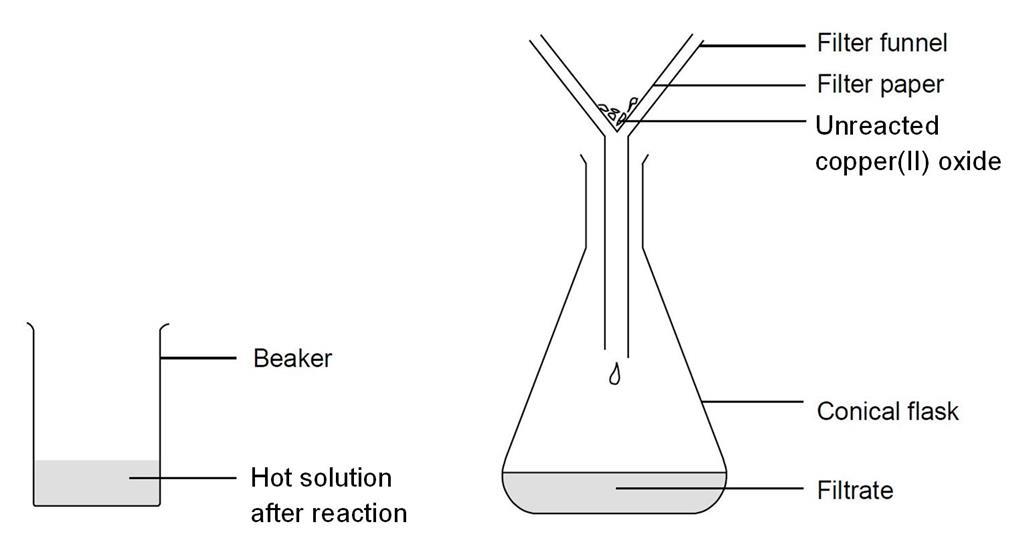
The diagram below shows a stage in the preparation of copper chloride Copper Oxide And Hydrochloric Acid Colour Change At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). Learn about basic oxides, acid. Copper oxide is black in colour. Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. The compound formed when copper oxide reacts with. Copper Oxide And Hydrochloric Acid Colour Change.
From www.youtube.com
How to Balance CuO + HCl = CuCl2 + H2O YouTube Copper Oxide And Hydrochloric Acid Colour Change When the two are mixed together, the solution turns green because the copper reacts with the acid to form. The solution of copper(ii) chloride is. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. Copper oxide is black in colour. At a guess, you're seeing the copper turn pink because it. Copper Oxide And Hydrochloric Acid Colour Change.
From loecqvsbo.blob.core.windows.net
Copper Hydroxide + Hydrochloric Acid at Joseph Keeble blog Copper Oxide And Hydrochloric Acid Colour Change The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). The solution of copper(ii) chloride is. When the two are mixed together, the solution turns green because the copper reacts with the acid to form. Learn about basic oxides, acid. Copper oxide is black in colour. When it reacts with dil.hcl, no reaction takes place. Copper Oxide And Hydrochloric Acid Colour Change.
From www.youtube.com
Reaction of Copper Oxide With Hydrochloric Acid YouTube Copper Oxide And Hydrochloric Acid Colour Change When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). Learn about basic oxides, acid. Copper oxide is black in colour.. Copper Oxide And Hydrochloric Acid Colour Change.
From www.youtube.com
neutralisation of sulphuric acid with copper oxide.mp4 YouTube Copper Oxide And Hydrochloric Acid Colour Change The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. When the two are mixed together, the solution turns green because the copper reacts with the acid to form. Learn about basic oxides, acid. The solution of. Copper Oxide And Hydrochloric Acid Colour Change.
From www.sciencephoto.com
Copper in hydrochloric acid Stock Image A500/0621 Science Photo Copper Oxide And Hydrochloric Acid Colour Change The solution of copper(ii) chloride is. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. Learn about basic oxides, acid. At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). Copper oxide is black in colour.. Copper Oxide And Hydrochloric Acid Colour Change.
From www.youtube.com
Type of Reaction for Cu + HCl (Copper + Hydrochloric acid) YouTube Copper Oxide And Hydrochloric Acid Colour Change When the two are mixed together, the solution turns green because the copper reacts with the acid to form. Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). Copper oxide is black in colour. The solution of copper(ii). Copper Oxide And Hydrochloric Acid Colour Change.
From www.numerade.com
SOLVED 6. Copper(II) oxide reacts with hydrochloric acid to produce Copper Oxide And Hydrochloric Acid Colour Change Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). The solution of copper(ii) chloride is. Learn about basic oxides, acid. Copper oxide is black in colour. When it reacts with dil.hcl, no reaction takes place as copper is. Copper Oxide And Hydrochloric Acid Colour Change.
From www.sciencephoto.com
Iron (III) oxide in water and in hydrochloric acid Stock Image C036 Copper Oxide And Hydrochloric Acid Colour Change Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. When the two are mixed together, the solution turns green because the copper reacts with the acid to form. Learn about basic oxides, acid. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. The solution. Copper Oxide And Hydrochloric Acid Colour Change.
From brainly.in
reaction between titanium oxide and sulphuric acid Brainly.in Copper Oxide And Hydrochloric Acid Colour Change Learn about basic oxides, acid. When the two are mixed together, the solution turns green because the copper reacts with the acid to form. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). The solution of. Copper Oxide And Hydrochloric Acid Colour Change.
From www.youtube.com
copper carbonate + hydrochloric acid YouTube Copper Oxide And Hydrochloric Acid Colour Change When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. Copper oxide is black in colour. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). The solution of copper(ii) chloride is. When the two are mixed together, the solution turns green because the copper reacts with. Copper Oxide And Hydrochloric Acid Colour Change.
From www.youtube.com
COPPER(II) CARBONATE & HYDROCHLORIC ACID DEMONSTRATION YouTube Copper Oxide And Hydrochloric Acid Colour Change The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). The solution of copper(ii) chloride is. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. At a guess, you're seeing. Copper Oxide And Hydrochloric Acid Colour Change.
From www.sciencephoto.com
Copper in hydrochloric acid Stock Image A500/0755 Science Photo Copper Oxide And Hydrochloric Acid Colour Change When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). Learn about basic oxides, acid. The solution of copper(ii) chloride is. Copper oxide is black in colour.. Copper Oxide And Hydrochloric Acid Colour Change.
From chemicaldb.netlify.app
Copper acid or base Copper Oxide And Hydrochloric Acid Colour Change Copper oxide is black in colour. At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). The solution of copper(ii) chloride is. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. Explore the reaction between copper. Copper Oxide And Hydrochloric Acid Colour Change.
From webapi.bu.edu
🏆 Copper carbonate experiment. What happens when copper carbonate Copper Oxide And Hydrochloric Acid Colour Change Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. Copper oxide is black in colour. When the two are mixed together, the solution turns green because the copper reacts with the acid to form. Learn about basic oxides, acid. When it reacts with dil.hcl, no reaction takes place as copper is less reactive. Copper Oxide And Hydrochloric Acid Colour Change.
From www.sciencephoto.com
Two copper oxide reactions Stock Image A500/0465 Science Photo Library Copper Oxide And Hydrochloric Acid Colour Change At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). Learn about basic oxides, acid. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than. Copper Oxide And Hydrochloric Acid Colour Change.
From www.youtube.com
Mixing copper oxide with distilled water and sulfuric acid MVI 6706 Copper Oxide And Hydrochloric Acid Colour Change When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. When the two are mixed together, the solution turns green because. Copper Oxide And Hydrochloric Acid Colour Change.
From www.toppr.com
On adding dilute hydrochloric acid to copper oxide powder, the solution Copper Oxide And Hydrochloric Acid Colour Change Copper oxide is black in colour. At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). The solution of copper(ii) chloride is. Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. Learn about basic oxides, acid. When it. Copper Oxide And Hydrochloric Acid Colour Change.
From www.sciencephoto.com
Iron (III) oxide in hydrochloric acid Stock Image C036/3132 Copper Oxide And Hydrochloric Acid Colour Change At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). The solution of copper(ii) chloride is. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). When it reacts with dil.hcl, no reaction takes place as copper is less reactive. Copper Oxide And Hydrochloric Acid Colour Change.
From www.youtube.com
Hydrochloric Acid Reaction HCL and mixed metal copper oxides YouTube Copper Oxide And Hydrochloric Acid Colour Change Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). Copper oxide is black in colour. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii). Copper Oxide And Hydrochloric Acid Colour Change.
From insende.netlify.app
30++ Hydrochloric Acid Burn Through Metal Insende Copper Oxide And Hydrochloric Acid Colour Change The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. Learn about basic oxides, acid. Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. Copper oxide is black in colour.. Copper Oxide And Hydrochloric Acid Colour Change.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Oxide And Hydrochloric Acid Colour Change When the two are mixed together, the solution turns green because the copper reacts with the acid to form. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). Explore the reaction between copper oxide and hydrochloric acid in this ncert class 10 science activity. Learn about basic oxides, acid. When it reacts with dil.hcl,. Copper Oxide And Hydrochloric Acid Colour Change.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Oxide And Hydrochloric Acid Colour Change At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). When the two are mixed together, the solution turns green because the copper reacts with the acid to form. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen. Copper Oxide And Hydrochloric Acid Colour Change.
From jaxkruwfletcher.blogspot.com
Copper Oxide Sulfuric Acid JaxkruwFletcher Copper Oxide And Hydrochloric Acid Colour Change At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). Learn about basic oxides, acid. The solution of copper(ii) chloride is. Copper oxide is black in colour. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). Explore the reaction. Copper Oxide And Hydrochloric Acid Colour Change.
From www.gkseries.com
When copper oxide and dilute hydrochloric acid react, colour changes to Copper Oxide And Hydrochloric Acid Colour Change When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. Learn about basic oxides, acid. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid,. Copper Oxide And Hydrochloric Acid Colour Change.
From brainly.in
Colour of Copper oxide? Hehehehe Brainly.in Copper Oxide And Hydrochloric Acid Colour Change Copper oxide is black in colour. The compound formed when copper oxide reacts with dilute hydrochloric acid is copper(ii) chloride (cucl₂). The solution of copper(ii) chloride is. When it reacts with dil.hcl, no reaction takes place as copper is less reactive than hydrogen and cannot. When the two are mixed together, the solution turns green because the copper reacts with. Copper Oxide And Hydrochloric Acid Colour Change.
From www.slideserve.com
PPT CHAPTER 6 PHYSICAL AND CHEMICAL CHANGES PowerPoint Presentation Copper Oxide And Hydrochloric Acid Colour Change At a guess, you're seeing the copper turn pink because it has a significant oxide layer that is reacting with the acid, forming copper (ii). Copper oxide is black in colour. When the two are mixed together, the solution turns green because the copper reacts with the acid to form. The compound formed when copper oxide reacts with dilute hydrochloric. Copper Oxide And Hydrochloric Acid Colour Change.