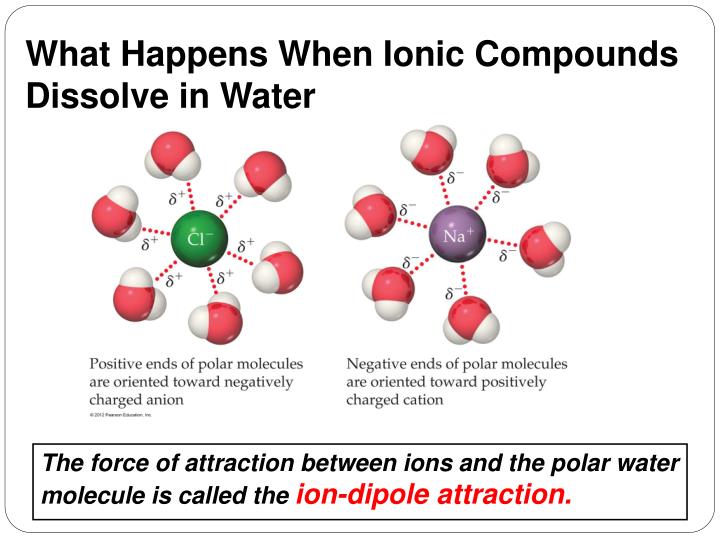
What Substances Can Dissolve In Water . Salt is an ionic compound that dissolves into a sodium ion (na +) and a chlorine ion (cl. The same forces of attraction among molecules. An aqueous solution is water that contains one or more dissolved substance. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. What this means is that water can act as both an acid and a base. One of the most important properties of liquid water is its ability to dissolve many different substances. Water is called the universal solvent because more substances dissolve in water than in any other chemical. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Amphoterism makes water a better solvent than most other polar molecules. This has to do with the polarity of each water molecule. Water can become so heavily attracted to a different compound, like salt (nacl), that it can disrupt the attractive forces that hold. For example, consider how ordinary table salt (nacl) dissolves in water. The dissolved substances in an aqueous solution.
from www.slideserve.com
The same forces of attraction among molecules. Amphoterism makes water a better solvent than most other polar molecules. For example, consider how ordinary table salt (nacl) dissolves in water. What this means is that water can act as both an acid and a base. An aqueous solution is water that contains one or more dissolved substance. This has to do with the polarity of each water molecule. One of the most important properties of liquid water is its ability to dissolve many different substances. The dissolved substances in an aqueous solution. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent.
PPT UNIT 5 PowerPoint Presentation ID2276550
What Substances Can Dissolve In Water What this means is that water can act as both an acid and a base. One of the most important properties of liquid water is its ability to dissolve many different substances. Water can become so heavily attracted to a different compound, like salt (nacl), that it can disrupt the attractive forces that hold. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. What this means is that water can act as both an acid and a base. This has to do with the polarity of each water molecule. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Water is called the universal solvent because more substances dissolve in water than in any other chemical. An aqueous solution is water that contains one or more dissolved substance. The dissolved substances in an aqueous solution. Amphoterism makes water a better solvent than most other polar molecules. The same forces of attraction among molecules. For example, consider how ordinary table salt (nacl) dissolves in water. Salt is an ionic compound that dissolves into a sodium ion (na +) and a chlorine ion (cl.
From highlands229.blogspot.com
Highlands Middle School 7th Grade Science 20192020 What Substances Can Dissolve In Water Water can become so heavily attracted to a different compound, like salt (nacl), that it can disrupt the attractive forces that hold. For example, consider how ordinary table salt (nacl) dissolves in water. One of the most important properties of liquid water is its ability to dissolve many different substances. Salt is an ionic compound that dissolves into a sodium. What Substances Can Dissolve In Water.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik What Substances Can Dissolve In Water Water can become so heavily attracted to a different compound, like salt (nacl), that it can disrupt the attractive forces that hold. An aqueous solution is water that contains one or more dissolved substance. The dissolved substances in an aqueous solution. Water is called the universal solvent because more substances dissolve in water than in any other chemical. Water, which. What Substances Can Dissolve In Water.
From littlebinsforlittlehands.com
What Dissolves In Water Experiment Little Bins for Little Hands What Substances Can Dissolve In Water What this means is that water can act as both an acid and a base. One of the most important properties of liquid water is its ability to dissolve many different substances. For example, consider how ordinary table salt (nacl) dissolves in water. The same forces of attraction among molecules. Water is called the universal solvent because more substances dissolve. What Substances Can Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Substances Can Dissolve In Water The dissolved substances in an aqueous solution. Water is called the universal solvent because more substances dissolve in water than in any other chemical. For example, consider how ordinary table salt (nacl) dissolves in water. This has to do with the polarity of each water molecule. Water can become so heavily attracted to a different compound, like salt (nacl), that. What Substances Can Dissolve In Water.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID What Substances Can Dissolve In Water For example, consider how ordinary table salt (nacl) dissolves in water. Amphoterism makes water a better solvent than most other polar molecules. An aqueous solution is water that contains one or more dissolved substance. Water is called the universal solvent because more substances dissolve in water than in any other chemical. What this means is that water can act as. What Substances Can Dissolve In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 What Substances Can Dissolve In Water The same forces of attraction among molecules. Amphoterism makes water a better solvent than most other polar molecules. The dissolved substances in an aqueous solution. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. What this means is that water can act as both an acid and a base.. What Substances Can Dissolve In Water.
From www.youtube.com
Which substances dissolve in water? YouTube What Substances Can Dissolve In Water Water is called the universal solvent because more substances dissolve in water than in any other chemical. This has to do with the polarity of each water molecule. One of the most important properties of liquid water is its ability to dissolve many different substances. The same forces of attraction among molecules. Amphoterism makes water a better solvent than most. What Substances Can Dissolve In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry What Substances Can Dissolve In Water For example, consider how ordinary table salt (nacl) dissolves in water. An aqueous solution is water that contains one or more dissolved substance. Amphoterism makes water a better solvent than most other polar molecules. This has to do with the polarity of each water molecule. Water is called the universal solvent because more substances dissolve in water than in any. What Substances Can Dissolve In Water.
From teachbesideme.com
Dissolving Science Experiment What Dissolves in Water? Teach Beside Me What Substances Can Dissolve In Water Salt is an ionic compound that dissolves into a sodium ion (na +) and a chlorine ion (cl. What this means is that water can act as both an acid and a base. For example, consider how ordinary table salt (nacl) dissolves in water. The same forces of attraction among molecules. Amphoterism makes water a better solvent than most other. What Substances Can Dissolve In Water.
From sciencenotes.org
Is Dissolving Salt in Water a Chemical Change or a Physical Change? What Substances Can Dissolve In Water One of the most important properties of liquid water is its ability to dissolve many different substances. An aqueous solution is water that contains one or more dissolved substance. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Water is called the universal solvent because more substances dissolve in. What Substances Can Dissolve In Water.
From sciencenotes.org
Why Is Water Called the Universal Solvent? What Substances Can Dissolve In Water The dissolved substances in an aqueous solution. Water is called the universal solvent because more substances dissolve in water than in any other chemical. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Amphoterism makes water a better solvent than most other polar molecules. This has to do with. What Substances Can Dissolve In Water.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? What Substances Can Dissolve In Water The same forces of attraction among molecules. Amphoterism makes water a better solvent than most other polar molecules. This has to do with the polarity of each water molecule. Salt is an ionic compound that dissolves into a sodium ion (na +) and a chlorine ion (cl. The dissolved substances in an aqueous solution. One of the most important properties. What Substances Can Dissolve In Water.
From www.slideserve.com
PPT Section 2 How Substances Dissolve PowerPoint Presentation, free What Substances Can Dissolve In Water One of the most important properties of liquid water is its ability to dissolve many different substances. This has to do with the polarity of each water molecule. For example, consider how ordinary table salt (nacl) dissolves in water. Amphoterism makes water a better solvent than most other polar molecules. What this means is that water can act as both. What Substances Can Dissolve In Water.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize What Substances Can Dissolve In Water What this means is that water can act as both an acid and a base. The same forces of attraction among molecules. An aqueous solution is water that contains one or more dissolved substance. Water is called the universal solvent because more substances dissolve in water than in any other chemical. Water, which not only dissolves many compounds but also. What Substances Can Dissolve In Water.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures What Substances Can Dissolve In Water Water is called the universal solvent because more substances dissolve in water than in any other chemical. Salt is an ionic compound that dissolves into a sodium ion (na +) and a chlorine ion (cl. An aqueous solution is water that contains one or more dissolved substance. Water, which not only dissolves many compounds but also dissolves more substances than. What Substances Can Dissolve In Water.
From www.slideserve.com
PPT Water, Water, Everywhere (Ch. 3) PowerPoint Presentation, free What Substances Can Dissolve In Water Water is called the universal solvent because more substances dissolve in water than in any other chemical. This has to do with the polarity of each water molecule. The dissolved substances in an aqueous solution. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Amphoterism makes water a. What Substances Can Dissolve In Water.
From brainly.in
The image shows particles of salt dissolved in water. Brainly.in What Substances Can Dissolve In Water One of the most important properties of liquid water is its ability to dissolve many different substances. Water can become so heavily attracted to a different compound, like salt (nacl), that it can disrupt the attractive forces that hold. What this means is that water can act as both an acid and a base. This has to do with the. What Substances Can Dissolve In Water.
From www.pinterest.com
8 Worksheets Dissolves In Water Science activities for kids, Science What Substances Can Dissolve In Water Water is called the universal solvent because more substances dissolve in water than in any other chemical. Amphoterism makes water a better solvent than most other polar molecules. The dissolved substances in an aqueous solution. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. For example, consider how ordinary. What Substances Can Dissolve In Water.
From publicaffairsworld.com
what dissolves in water science project What Substances Can Dissolve In Water The dissolved substances in an aqueous solution. An aqueous solution is water that contains one or more dissolved substance. Water can become so heavily attracted to a different compound, like salt (nacl), that it can disrupt the attractive forces that hold. The same forces of attraction among molecules. One of the most important properties of liquid water is its ability. What Substances Can Dissolve In Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids What Substances Can Dissolve In Water Water can become so heavily attracted to a different compound, like salt (nacl), that it can disrupt the attractive forces that hold. This has to do with the polarity of each water molecule. One of the most important properties of liquid water is its ability to dissolve many different substances. The same forces of attraction among molecules. The dissolved substances. What Substances Can Dissolve In Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids What Substances Can Dissolve In Water Salt is an ionic compound that dissolves into a sodium ion (na +) and a chlorine ion (cl. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. For example, consider how ordinary table salt (nacl) dissolves in water. Water, which not only dissolves many compounds but also dissolves more. What Substances Can Dissolve In Water.
From aceparents.com
Easy Preschool Science Experiment to Learn What Dissolves in Water What Substances Can Dissolve In Water Water can become so heavily attracted to a different compound, like salt (nacl), that it can disrupt the attractive forces that hold. The dissolved substances in an aqueous solution. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Amphoterism makes water a better solvent than most other polar molecules.. What Substances Can Dissolve In Water.
From study.com
Compound Solubility in Water Overview & Examples Lesson What Substances Can Dissolve In Water An aqueous solution is water that contains one or more dissolved substance. Water can become so heavily attracted to a different compound, like salt (nacl), that it can disrupt the attractive forces that hold. Water is called the universal solvent because more substances dissolve in water than in any other chemical. Salt is an ionic compound that dissolves into a. What Substances Can Dissolve In Water.
From www.ehow.com
What Happens When a Substance Dissolves in Water? Sciencing What Substances Can Dissolve In Water The dissolved substances in an aqueous solution. Salt is an ionic compound that dissolves into a sodium ion (na +) and a chlorine ion (cl. One of the most important properties of liquid water is its ability to dissolve many different substances. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields. What Substances Can Dissolve In Water.
From teachbesideme.com
Dissolving Science Experiment What Dissolves in Water? Teach Beside Me What Substances Can Dissolve In Water One of the most important properties of liquid water is its ability to dissolve many different substances. Salt is an ionic compound that dissolves into a sodium ion (na +) and a chlorine ion (cl. For example, consider how ordinary table salt (nacl) dissolves in water. What this means is that water can act as both an acid and a. What Substances Can Dissolve In Water.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID What Substances Can Dissolve In Water For example, consider how ordinary table salt (nacl) dissolves in water. What this means is that water can act as both an acid and a base. One of the most important properties of liquid water is its ability to dissolve many different substances. An aqueous solution is water that contains one or more dissolved substance. Water, which not only dissolves. What Substances Can Dissolve In Water.
From www.thoughtco.com
Dissolve Definition in Chemistry What Substances Can Dissolve In Water Water is called the universal solvent because more substances dissolve in water than in any other chemical. An aqueous solution is water that contains one or more dissolved substance. What this means is that water can act as both an acid and a base. One of the most important properties of liquid water is its ability to dissolve many different. What Substances Can Dissolve In Water.
From gracelimlf.blogspot.com
LIM LAI FONG (D20102043844) Science Year 3 What Substances Can Dissolve In Water Salt is an ionic compound that dissolves into a sodium ion (na +) and a chlorine ion (cl. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. The dissolved substances in an aqueous solution. This has to do with the polarity of each water molecule. The same forces of. What Substances Can Dissolve In Water.
From www.slideserve.com
PPT Part I Solubility, Factors Affecting Solubility PowerPoint What Substances Can Dissolve In Water The same forces of attraction among molecules. An aqueous solution is water that contains one or more dissolved substance. For example, consider how ordinary table salt (nacl) dissolves in water. Water is called the universal solvent because more substances dissolve in water than in any other chemical. What this means is that water can act as both an acid and. What Substances Can Dissolve In Water.
From courses.lumenlearning.com
The Dissolution Process Chemistry What Substances Can Dissolve In Water This has to do with the polarity of each water molecule. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Water is called the universal solvent because more substances dissolve in water than in any other chemical. What this means is that water can act as both an. What Substances Can Dissolve In Water.
From www.chegg.com
Solved 2. The following compounds dissolve readily in water. What Substances Can Dissolve In Water Water is called the universal solvent because more substances dissolve in water than in any other chemical. What this means is that water can act as both an acid and a base. This has to do with the polarity of each water molecule. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. What Substances Can Dissolve In Water.
From www.nagwa.com
Question Video Identifying the Type of Substance Formed When a What Substances Can Dissolve In Water When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Amphoterism makes water a better solvent than most other polar molecules. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Water is called the universal solvent because more. What Substances Can Dissolve In Water.
From www.youtube.com
Can Liquids Dissolve in Water? YouTube What Substances Can Dissolve In Water Amphoterism makes water a better solvent than most other polar molecules. This has to do with the polarity of each water molecule. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. One of the most important properties of liquid water is its ability to dissolve many different substances. An. What Substances Can Dissolve In Water.
From byjus.com
Which of the given substances will dissolve in water? What Substances Can Dissolve In Water This has to do with the polarity of each water molecule. For example, consider how ordinary table salt (nacl) dissolves in water. Amphoterism makes water a better solvent than most other polar molecules. What this means is that water can act as both an acid and a base. When some substances are dissolved in water, they undergo either a physical. What Substances Can Dissolve In Water.
From littlebinsforlittlehands.com
Which Solids Dissolve In Water Chemistry for Kids What Substances Can Dissolve In Water Water is called the universal solvent because more substances dissolve in water than in any other chemical. An aqueous solution is water that contains one or more dissolved substance. This has to do with the polarity of each water molecule. The same forces of attraction among molecules. Water, which not only dissolves many compounds but also dissolves more substances than. What Substances Can Dissolve In Water.