Difference Between Electrolytic And Voltaic Cell . Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. Voltaic cells are used to charge batteries. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. Voltaic cells produce electricity, while electrolytic cells use a power. Voltaic cell what's the difference? Electrolytic cells and voltaic cells are both types of electrochemical cells used to. The main difference between voltaic and electrolytic cells is their purpose. Electrolytic cells, on the other hand, are used to.
from pediaa.com
A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. Voltaic cells are used to charge batteries. Voltaic cells produce electricity, while electrolytic cells use a power. The main difference between voltaic and electrolytic cells is their purpose. Electrolytic cells, on the other hand, are used to. Electrolytic cells and voltaic cells are both types of electrochemical cells used to. Voltaic cell what's the difference?
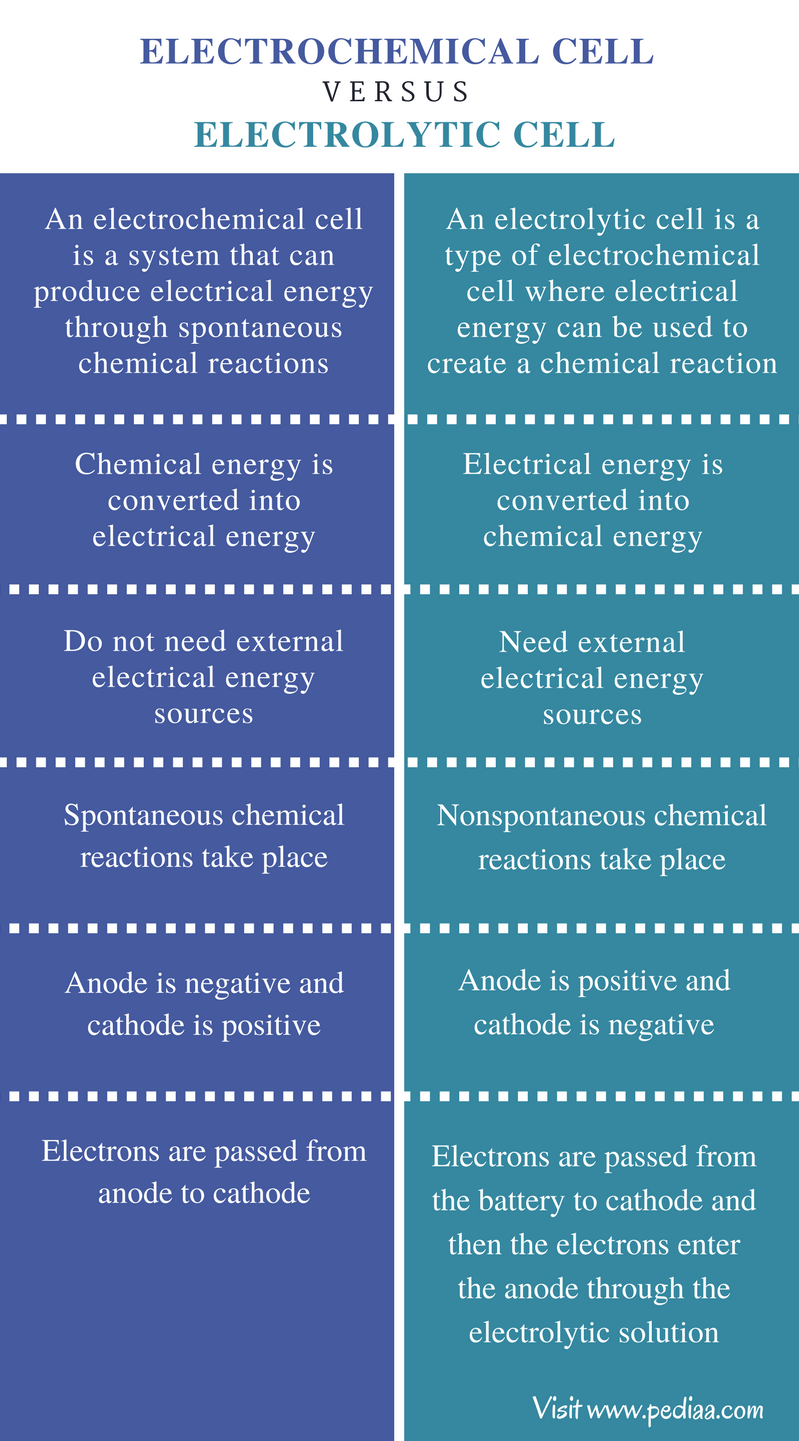
Difference Between Electrochemical Cell and Electrolytic Cell
Difference Between Electrolytic And Voltaic Cell Voltaic cells are used to charge batteries. Voltaic cell what's the difference? Voltaic cells are used to charge batteries. Electrolytic cells and voltaic cells are both types of electrochemical cells used to. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. Electrolytic cells, on the other hand, are used to. Voltaic cells produce electricity, while electrolytic cells use a power. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The main difference between voltaic and electrolytic cells is their purpose.
From www.javatpoint.com
Difference Between Galvanic Cells and Electrolytic Cells javatpoint Difference Between Electrolytic And Voltaic Cell Voltaic cells are used to charge batteries. Electrolytic cells and voltaic cells are both types of electrochemical cells used to. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. Voltaic cells produce electricity, while electrolytic cells use a power. Voltaic cell what's. Difference Between Electrolytic And Voltaic Cell.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID2330730 Difference Between Electrolytic And Voltaic Cell The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons.. Difference Between Electrolytic And Voltaic Cell.
From www.chegg.com
Solved What is the difference between a voltaic cell and an Difference Between Electrolytic And Voltaic Cell The main difference between voltaic and electrolytic cells is their purpose. Electrolytic cells, on the other hand, are used to. Voltaic cells are used to charge batteries. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. Voltaic cell what's the difference? Electrolytic. Difference Between Electrolytic And Voltaic Cell.
From www.pinterest.com
What are the differences between Galvanic cell and Electrolytic cell Difference Between Electrolytic And Voltaic Cell Electrolytic cells, on the other hand, are used to. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. Difference Between Electrolytic And Voltaic Cell.
From www.slideserve.com
PPT Electrolytic Cells Notes PowerPoint Presentation, free download Difference Between Electrolytic And Voltaic Cell The main difference between voltaic and electrolytic cells is their purpose. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to. Difference Between Electrolytic And Voltaic Cell.
From differguide.com
Difference Between Galvanic And Electrolytic Cell Difference Between Electrolytic And Voltaic Cell Voltaic cells are used to charge batteries. Electrolytic cells and voltaic cells are both types of electrochemical cells used to. Voltaic cells produce electricity, while electrolytic cells use a power. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Electrolytic cells, on the other hand, are used to.. Difference Between Electrolytic And Voltaic Cell.
From www.youtube.com
The differences between the voltaic cell and the electrolytic cell Difference Between Electrolytic And Voltaic Cell Electrolytic cells, on the other hand, are used to. Voltaic cell what's the difference? Electrolytic cells and voltaic cells are both types of electrochemical cells used to. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons.. Difference Between Electrolytic And Voltaic Cell.
From www.youtube.com
9.2 Comparison of electrochemical cells (SL) YouTube Difference Between Electrolytic And Voltaic Cell Voltaic cell what's the difference? A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Voltaic cells are used to charge batteries. The main difference between voltaic and electrolytic cells is their purpose. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to. Difference Between Electrolytic And Voltaic Cell.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Difference Between Electrolytic And Voltaic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. Voltaic cells are used to charge batteries. Electrolytic cells and voltaic cells are both types of electrochemical cells used to. Electrolytic cells, on the other hand, are. Difference Between Electrolytic And Voltaic Cell.
From kayleyewabarr.blogspot.com
Anode and Cathode in Electrolysis KayleyewaBarr Difference Between Electrolytic And Voltaic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. Voltaic cells are used to charge batteries. Electrolytic cells, on the other hand, are used to. The main difference between voltaic cell and electrolytic cell is that. Difference Between Electrolytic And Voltaic Cell.
From www.artofit.org
Electrochemical cell Artofit Difference Between Electrolytic And Voltaic Cell A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. Voltaic cells produce electricity, while electrolytic cells use a power. Voltaic cells. Difference Between Electrolytic And Voltaic Cell.
From slidetodoc.com
Electrochemistry Spontaneity of Redox Reactions 21 1 Electrochemistry Difference Between Electrolytic And Voltaic Cell Electrolytic cells, on the other hand, are used to. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. The main difference between voltaic and electrolytic cells is their purpose. Voltaic cells are used to charge batteries. Electrolytic cells and voltaic cells are. Difference Between Electrolytic And Voltaic Cell.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Difference Between Electrolytic And Voltaic Cell Electrolytic cells, on the other hand, are used to. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. Voltaic cells are used to charge batteries. The main difference between voltaic and electrolytic cells is their purpose.. Difference Between Electrolytic And Voltaic Cell.
From www.shutterstock.com
Voltaic Cells Vs Electrolytic Cells Stock Vector (Royalty Free Difference Between Electrolytic And Voltaic Cell Electrolytic cells and voltaic cells are both types of electrochemical cells used to. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The main difference between voltaic and electrolytic cells is their purpose. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require. Difference Between Electrolytic And Voltaic Cell.
From www.vcalc.com
CHM1 20 Electrolytic Cells Collection Difference Between Electrolytic And Voltaic Cell Voltaic cell what's the difference? A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Electrolytic cells, on the other hand, are used to. Electrolytic cells and voltaic cells are both types of electrochemical cells used to. Voltaic cells produce electricity, while electrolytic cells use a power. The main. Difference Between Electrolytic And Voltaic Cell.
From www.coursehero.com
[Solved] differentiate voltaic cell and electrolytic cell. tabulate Difference Between Electrolytic And Voltaic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. Voltaic cells are used to charge batteries. Electrolytic cells, on the other hand, are used to. The main difference between voltaic cell and electrolytic cell is that. Difference Between Electrolytic And Voltaic Cell.
From chem.libretexts.org
Voltaic Cells Chemistry LibreTexts Difference Between Electrolytic And Voltaic Cell Electrolytic cells and voltaic cells are both types of electrochemical cells used to. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. Voltaic cell what's the difference? Electrolytic cells, on the other hand, are used to. Voltaic cells produce electricity, while electrolytic. Difference Between Electrolytic And Voltaic Cell.
From www.differencebetween.net
Difference Between Galvanic Cells and Electrolytic Cells Difference Difference Between Electrolytic And Voltaic Cell The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. The main difference between voltaic and electrolytic cells is their purpose. Voltaic cells are used to charge batteries. Voltaic cells produce electricity, while electrolytic cells use a power. A voltaic cell (also known. Difference Between Electrolytic And Voltaic Cell.
From www.youtube.com
Difference Between Galvanic or Voltaic Cell and Electrolytic Cell YouTube Difference Between Electrolytic And Voltaic Cell Electrolytic cells and voltaic cells are both types of electrochemical cells used to. Voltaic cell what's the difference? Voltaic cells are used to charge batteries. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. The main. Difference Between Electrolytic And Voltaic Cell.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID Difference Between Electrolytic And Voltaic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. The main difference between voltaic and electrolytic cells is their purpose. Electrolytic cells, on the other hand, are used to. A voltaic cell (also known as a. Difference Between Electrolytic And Voltaic Cell.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell Difference Between Electrolytic And Voltaic Cell Electrolytic cells, on the other hand, are used to. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions. Difference Between Electrolytic And Voltaic Cell.
From www.differencebetween.com
What is the Difference Between Voltaic Cell and Electrolytic Cell Difference Between Electrolytic And Voltaic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. Voltaic cells are used to charge batteries. Voltaic cell what's the difference? A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses. Difference Between Electrolytic And Voltaic Cell.
From psiberg.com
Galvanic vs. Electrolytic Cell The Two Types of Electrochemical Cells Difference Between Electrolytic And Voltaic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The main difference between voltaic cell. Difference Between Electrolytic And Voltaic Cell.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Difference Between Electrolytic And Voltaic Cell A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The main difference between voltaic and electrolytic cells is their purpose. Voltaic cells produce electricity, while electrolytic cells use a power. Electrolytic cells and voltaic cells are both types of electrochemical cells used to. Voltaic cell what's the difference?. Difference Between Electrolytic And Voltaic Cell.
From exotyizys.blob.core.windows.net
What Is The Difference Between Electrochemical And Electrolytic Cell at Difference Between Electrolytic And Voltaic Cell Electrolytic cells and voltaic cells are both types of electrochemical cells used to. Voltaic cell what's the difference? Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons. Voltaic cells produce electricity, while electrolytic cells use a. Difference Between Electrolytic And Voltaic Cell.
From www.numerade.com
SOLVED Taking my DAT in 3 days. Whats the most major differences Difference Between Electrolytic And Voltaic Cell Voltaic cells produce electricity, while electrolytic cells use a power. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. Voltaic cell what's the difference? A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions. Difference Between Electrolytic And Voltaic Cell.
From exotyizys.blob.core.windows.net
What Is The Difference Between Electrochemical And Electrolytic Cell at Difference Between Electrolytic And Voltaic Cell Electrolytic cells, on the other hand, are used to. Voltaic cells are used to charge batteries. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox. Difference Between Electrolytic And Voltaic Cell.
From brainly.in
What is the difference between galvanic cell and daniel cell Brainly.in Difference Between Electrolytic And Voltaic Cell A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Voltaic cells are used to charge batteries. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. The main difference between voltaic. Difference Between Electrolytic And Voltaic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Difference Between Electrolytic And Voltaic Cell Voltaic cells produce electricity, while electrolytic cells use a power. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. Voltaic cell what's the difference? A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions. Difference Between Electrolytic And Voltaic Cell.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Difference Between Electrolytic And Voltaic Cell The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The main difference between voltaic and electrolytic cells is their purpose. Electrolytic. Difference Between Electrolytic And Voltaic Cell.
From lavelle.chem.ucla.edu
Galvanic vs Voltaic Cells CHEMISTRY COMMUNITY Difference Between Electrolytic And Voltaic Cell Electrolytic cells, on the other hand, are used to. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode. Difference Between Electrolytic And Voltaic Cell.
From www.differencebetween.com
What is the Difference Between Voltaic Cell and Electrolytic Cell Difference Between Electrolytic And Voltaic Cell Voltaic cells are used to charge batteries. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Electrolytic cells and voltaic cells are both types of electrochemical cells used to. The main difference between voltaic and electrolytic cells is their purpose. Voltaic cells produce electricity, while electrolytic cells use. Difference Between Electrolytic And Voltaic Cell.
From www.youtube.com
Comparison between the electrolytic cell and chemical cell YouTube Difference Between Electrolytic And Voltaic Cell Voltaic cells are used to charge batteries. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Electrolytic cells and voltaic cells are both types of electrochemical cells used to. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both. Difference Between Electrolytic And Voltaic Cell.
From study.com
Voltaic vs. Electrolytic Cell Similarities, Differences & Uses Difference Between Electrolytic And Voltaic Cell Electrolytic cells, on the other hand, are used to. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to convert chemical energy into electrical energy, whereas an electrolytic cell is. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode. Difference Between Electrolytic And Voltaic Cell.
From overallscience.com
Voltaic Cell, Its Construction and Defects Overall Science Difference Between Electrolytic And Voltaic Cell Voltaic cells produce electricity, while electrolytic cells use a power. Electrolytic cells and voltaic cells are both types of electrochemical cells used to. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The main difference between voltaic cell and electrolytic cell is that voltaic cell is designed to. Difference Between Electrolytic And Voltaic Cell.