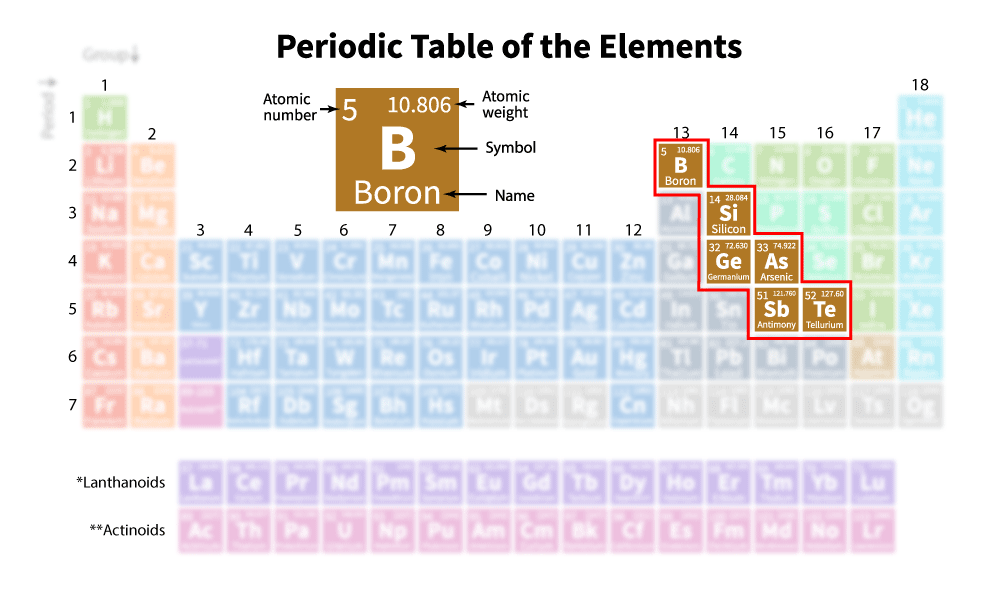
Are Metalloids Diatomic . The metalloid group separates the metals from the nonmetals. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Let’s learn what they are, and how they are different from diatomic molecules. Elements to the left are metals and nonmetals are to the. The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. Metalloids can also be called semimetals. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. There are seven diatomic elements: Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine,. On the periodic table, the elements. The periodic table has several diatomic elements, sometimes known as “molecular elements”. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. This periodic table shows the three different groups of elements.
from www.geeksforgeeks.org
On the periodic table, the elements. The periodic table has several diatomic elements, sometimes known as “molecular elements”. Metalloids can also be called semimetals. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Elements to the left are metals and nonmetals are to the. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine,. This periodic table shows the three different groups of elements. Let’s learn what they are, and how they are different from diatomic molecules. The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. The metalloid group separates the metals from the nonmetals.
Metalloids Definition, Position in Periodic Table, & Properties
Are Metalloids Diatomic There are seven diatomic elements: The metalloid group separates the metals from the nonmetals. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. Let’s learn what they are, and how they are different from diatomic molecules. The periodic table has several diatomic elements, sometimes known as “molecular elements”. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. Elements to the left are metals and nonmetals are to the. On the periodic table, the elements. This periodic table shows the three different groups of elements. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine,. There are seven diatomic elements: Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Metalloids can also be called semimetals.
From slideplayer.com
Chapter 3 Classification of Matter Elements Distribution, Names Are Metalloids Diatomic A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. There are seven diatomic elements: This periodic table shows the three different groups of elements. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine,. Are Metalloids Diatomic.
From thechemistrynotes.com
Diatomic Elements Important 7 Elements properties formation Are Metalloids Diatomic The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. The periodic table has several diatomic elements, sometimes known as “molecular elements”. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Let’s learn. Are Metalloids Diatomic.
From wisc.pb.unizin.org
D1.4 The Periodic Table Chemistry 109 Fall 2021 Are Metalloids Diatomic Elements to the left are metals and nonmetals are to the. The metalloid group separates the metals from the nonmetals. There are seven diatomic elements: For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. Let’s learn what they are, and how they are different from diatomic molecules.. Are Metalloids Diatomic.
From courses.lumenlearning.com
Periodicity Chemistry Are Metalloids Diatomic Let’s learn what they are, and how they are different from diatomic molecules. This periodic table shows the three different groups of elements. Elements to the left are metals and nonmetals are to the. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. The metalloid group separates the metals from the nonmetals. On the periodic. Are Metalloids Diatomic.
From www.slideserve.com
PPT The Atom PowerPoint Presentation, free download ID1375061 Are Metalloids Diatomic Metalloids can also be called semimetals. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. There are seven diatomic elements: This periodic table shows the three different groups of elements. The periodic table has several diatomic elements, sometimes known as “molecular elements”. A metalloid is an element that has properties that are intermediate between those. Are Metalloids Diatomic.
From sciencenotes.org
List of Metalloids or Semimetals Are Metalloids Diatomic Let’s learn what they are, and how they are different from diatomic molecules. On the periodic table, the elements. Metalloids can also be called semimetals. There are seven diatomic elements: The metalloid group separates the metals from the nonmetals. The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules. Are Metalloids Diatomic.
From sciencenotes.org
What Are the 7 Diatomic Elements? Definition and List Are Metalloids Diatomic Elements to the left are metals and nonmetals are to the. Metalloids can also be called semimetals. Let’s learn what they are, and how they are different from diatomic molecules. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Diatomic elements are pure elements that form molecules consisting of two atoms bonded. Are Metalloids Diatomic.
From www.slideserve.com
PPT Dalton’s Atomic Theory PowerPoint Presentation, free download Are Metalloids Diatomic The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. On the periodic table, the elements. Elements to the left are metals and nonmetals are to the. Let’s learn what they are, and how they are different from. Are Metalloids Diatomic.
From exozzfrsb.blob.core.windows.net
Metalloids Are Two Elements at Scott Hickson blog Are Metalloids Diatomic The metalloid group separates the metals from the nonmetals. This periodic table shows the three different groups of elements. Let’s learn what they are, and how they are different from diatomic molecules. There are seven diatomic elements: The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2,. Are Metalloids Diatomic.
From www.xometry.com
7 Elements of Metalloids Differences and Uses Xometry Are Metalloids Diatomic For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. The metalloid group separates the metals from the nonmetals. Metalloids can also be called semimetals. The periodic table has several diatomic elements, sometimes known as “molecular elements”. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine,. The noble gases are all. Are Metalloids Diatomic.
From thechemistrynotes.com
Metalloids Definition, Properties, Uses, and Applications Are Metalloids Diatomic The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. The periodic table has several diatomic elements, sometimes known as “molecular elements”. The metalloid group separates the metals from the nonmetals. Diatomic elements are pure elements that form. Are Metalloids Diatomic.
From sciencenotes.org
Metalloids Science Notes and Projects Are Metalloids Diatomic For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. There are seven diatomic elements: The periodic table has several diatomic elements, sometimes known as “molecular elements”. This periodic table shows the three different groups of elements. A metalloid is an element that has properties that are intermediate. Are Metalloids Diatomic.
From www.haikudeck.com
Metalloids by Megan Maul Are Metalloids Diatomic A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules. Are Metalloids Diatomic.
From slideplayer.com
Metals, Nonmetals and Metalloids ppt download Are Metalloids Diatomic Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine,. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Metalloids can also be called semimetals. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally. Are Metalloids Diatomic.
From slideplayer.com
Chapter 2 Atoms, Molecules, and Ions ppt download Are Metalloids Diatomic Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Let’s learn what they are, and how they are different from diatomic molecules. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they. Are Metalloids Diatomic.
From pediabay.com
Metalloids of the Periodic Table Pediabay Are Metalloids Diatomic The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. On the periodic table, the elements. The periodic table has several diatomic elements, sometimes known as “molecular elements”. Let’s learn what they are, and how they are different. Are Metalloids Diatomic.
From periodictableguide.com
Periodic table labeled with Metals Nonmetals and Metalloids Are Metalloids Diatomic The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. On the periodic table, the elements. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine,. Let’s learn what they are, and how they are different from diatomic molecules. Elements to the. Are Metalloids Diatomic.
From knordslearning.com
Metalloids Periodic Table (With Images) Are Metalloids Diatomic Elements to the left are metals and nonmetals are to the. The periodic table has several diatomic elements, sometimes known as “molecular elements”. Let’s learn what they are, and how they are different from diatomic molecules. There are seven diatomic elements: Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. The noble gases are all. Are Metalloids Diatomic.
From slideplayer.com
Periodic Table & Periodic Trends ppt download Are Metalloids Diatomic There are seven diatomic elements: On the periodic table, the elements. Elements to the left are metals and nonmetals are to the. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. A metalloid. Are Metalloids Diatomic.
From slideplayer.com
Chapter 3 Classification of Matter Elements Distribution, Names Are Metalloids Diatomic Let’s learn what they are, and how they are different from diatomic molecules. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. The metalloid group separates the metals from the nonmetals. Metalloids can also be called semimetals. The. Are Metalloids Diatomic.
From scientifictutor.org
Chem Further Divisions of the Periodic Table Scientific Tutor Are Metalloids Diatomic Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine,. On the periodic table, the elements. The periodic table has several diatomic elements, sometimes known as “molecular elements”. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the. Are Metalloids Diatomic.
From utedzz.blogspot.com
Periodic Table With Metals Metalloids And Nonmetals Labeled Periodic Are Metalloids Diatomic For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. The periodic table has several diatomic elements, sometimes known as “molecular elements”. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Diatomic elements are pure elements that form molecules consisting. Are Metalloids Diatomic.
From quizlet.com
Metals/NonMetals/Metalloids and Diatomic Elements Diagram Quizlet Are Metalloids Diatomic The periodic table has several diatomic elements, sometimes known as “molecular elements”. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. There are seven diatomic elements: Metalloids can also be called semimetals. A. Are Metalloids Diatomic.
From ar.inspiredpencil.com
Periodic Table With Metals Metalloids And Nonmetals Labeled Are Metalloids Diatomic The periodic table has several diatomic elements, sometimes known as “molecular elements”. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. There are seven diatomic elements: The metalloid group separates the metals from the nonmetals. The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist. Are Metalloids Diatomic.
From www.geeksforgeeks.org
Metalloids Definition, Position in Periodic Table, & Properties Are Metalloids Diatomic The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. The metalloid group separates the metals from the nonmetals. The periodic table has several diatomic elements, sometimes known as “molecular elements”. On the periodic table, the elements. A. Are Metalloids Diatomic.
From exozzfrsb.blob.core.windows.net
Metalloids Are Two Elements at Scott Hickson blog Are Metalloids Diatomic A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Elements to the left are metals and nonmetals are to the. The metalloid group separates the metals from the nonmetals. On the periodic table, the elements. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Metalloids can also. Are Metalloids Diatomic.
From www.slideserve.com
PPT Types of Chemical Bonds PowerPoint Presentation, free download Are Metalloids Diatomic Elements to the left are metals and nonmetals are to the. Metalloids can also be called semimetals. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. The noble gases are all monatomic, whereas. Are Metalloids Diatomic.
From www.adda247.com
What are Metalloids? Definition, Properties and Example Are Metalloids Diatomic The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. Elements to the left are metals. Are Metalloids Diatomic.
From www.slideserve.com
PPT Chemistry Chapter 5 PowerPoint Presentation, free download ID Are Metalloids Diatomic A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine,. This periodic table shows the three different groups of elements. There are seven diatomic elements: The periodic table has several diatomic elements, sometimes known as “molecular elements”. Metalloids can also be called semimetals. On the periodic table,. Are Metalloids Diatomic.
From elchoroukhost.net
List Of Representative Elements On The Periodic Table Elcho Table Are Metalloids Diatomic Let’s learn what they are, and how they are different from diatomic molecules. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine,. There are seven diatomic elements: Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Metalloids can also be called semimetals. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they. Are Metalloids Diatomic.
From slideplayer.com
Metals, Nonmetals, and Metalloids ppt download Are Metalloids Diatomic There are seven diatomic elements: The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. The periodic table has several diatomic elements, sometimes known as “molecular elements”. For example, the pure metalloids form covalent crystals like the nonmetals,. Are Metalloids Diatomic.
From byjus.com
Name all the metalloids Are Metalloids Diatomic The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. The metalloid group separates the metals from the nonmetals. There are seven diatomic elements: Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine,. Diatomic elements are pure elements that form molecules. Are Metalloids Diatomic.
From www.etutorworld.com
Molecular Geometry Chart Definition, Types, Examples & FAQs Are Metalloids Diatomic Metalloids can also be called semimetals. Elements to the left are metals and nonmetals are to the. There are seven diatomic elements: Let’s learn what they are, and how they are different from diatomic molecules. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen,. Are Metalloids Diatomic.
From utedzz.blogspot.com
Periodic Table Metalloids Periodic Table Timeline Are Metalloids Diatomic A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. There are seven diatomic elements: On the periodic table, the elements. This periodic table shows the three different groups of elements.. Are Metalloids Diatomic.
From edutechspot.com
Metalloids are located where on the periodic table? Here >>> Are Metalloids Diatomic The metalloid group separates the metals from the nonmetals. The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n 2, o 2, f 2, and cl 2. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine,. The periodic table has several diatomic elements, sometimes known as “molecular elements”. Elements. Are Metalloids Diatomic.