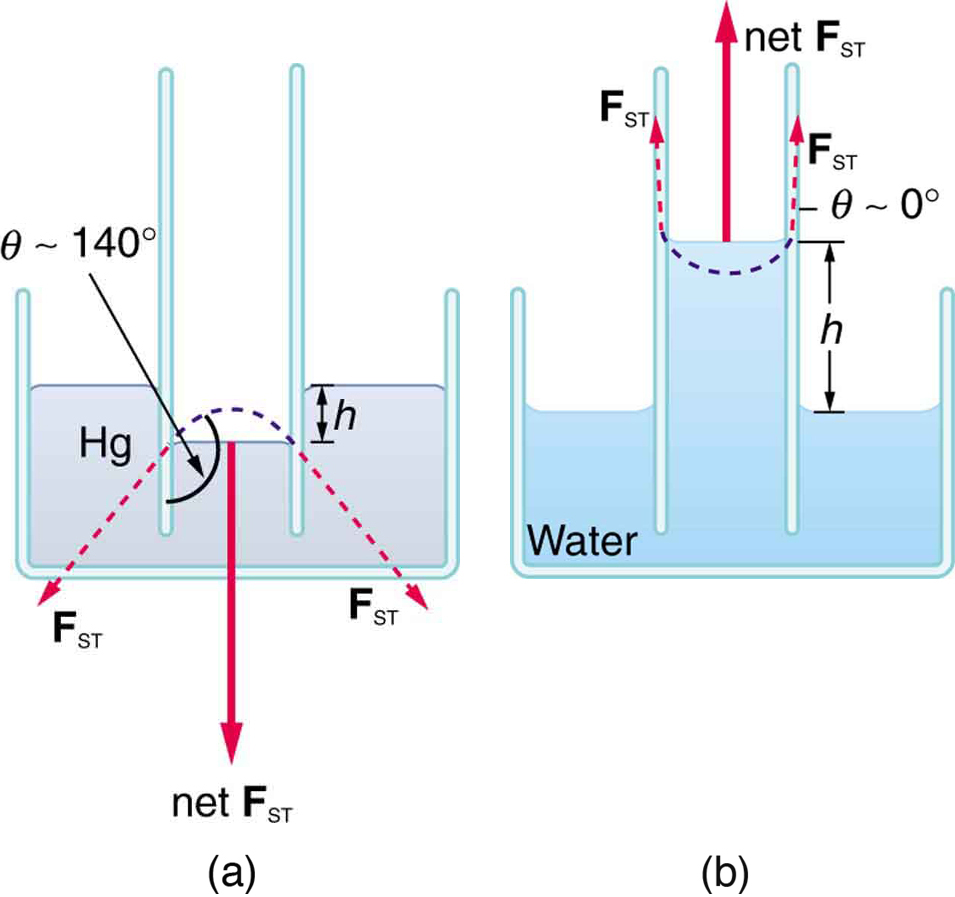
Liquid Surface Tension Effect . Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. For the analysis of the surface tension effects for. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause the. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Since these intermolecular forces vary depending on the nature of the. Attractive forces between molecules of different types are called adhesive forces.
from pressbooks.uiowa.edu
For the analysis of the surface tension effects for. Cohesive forces between molecules cause the. Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Attractive forces between molecules of different types are called adhesive forces.
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary
Liquid Surface Tension Effect Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Cohesive forces between molecules cause the. For the analysis of the surface tension effects for. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Attractive forces between molecules of different types are called adhesive forces. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Since these intermolecular forces vary depending on the nature of the.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID9168408 Liquid Surface Tension Effect For the analysis of the surface tension effects for. Attractive forces between molecules of different types are called adhesive forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. In the absence of other forces, the. Liquid Surface Tension Effect.
From pressbooks.online.ucf.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Liquid Surface Tension Effect In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. For the analysis of the surface tension effects for. Since these intermolecular forces vary depending on the nature of the. Surface tension is. Liquid Surface Tension Effect.
From pressbooks.uiowa.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Liquid Surface Tension Effect Attractive forces between molecules of different types are called adhesive forces. For the analysis of the surface tension effects for. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension. Liquid Surface Tension Effect.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint Liquid Surface Tension Effect Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Since these intermolecular forces vary depending on the nature of the. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. For the analysis of the surface tension effects for. In the absence of other forces, the surface tension. Liquid Surface Tension Effect.
From www.researchgate.net
Surface tension force at the gas/liquid interface of a bubble Liquid Surface Tension Effect In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Cohesive forces between molecules cause the. For the analysis of the surface tension effects for. Surface tension is defined as the energy required. Liquid Surface Tension Effect.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Liquid Surface Tension Effect Attractive forces between molecules of different types are called adhesive forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause the. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension is defined as. Liquid Surface Tension Effect.
From www.researchgate.net
The role of a low surface tension liquid on the droplet wetting state Liquid Surface Tension Effect Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Attractive forces between molecules of different types are called adhesive forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is proportional to the strength of the cohesive force, which varies with the type. Liquid Surface Tension Effect.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Liquid Surface Tension Effect Cohesive forces between molecules cause the. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension is the energy, or work, required to increase the surface. Liquid Surface Tension Effect.
From www.researchgate.net
(PDF) Surface Tension Effect of Core Liquid Surface Tension on the Liquid Surface Tension Effect In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. For the analysis. Liquid Surface Tension Effect.
From www.researchgate.net
Influence of the liquid surface tension on mean velocity (a, b and c Liquid Surface Tension Effect Cohesive forces between molecules cause the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Attractive forces. Liquid Surface Tension Effect.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free Liquid Surface Tension Effect Since these intermolecular forces vary depending on the nature of the. Attractive forces between molecules of different types are called adhesive forces. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\). Liquid Surface Tension Effect.
From www.youtube.com
Surface Tension Super Ability Of Liquid Water on Penny Experiment Liquid Surface Tension Effect Attractive forces between molecules of different types are called adhesive forces. Since these intermolecular forces vary depending on the nature of the. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\). Liquid Surface Tension Effect.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Liquid Surface Tension Effect Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Attractive forces between molecules of different types are called adhesive forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due. Liquid Surface Tension Effect.
From byjus.com
Explain the surface tension phenomenon with examples. Liquid Surface Tension Effect Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Since these intermolecular forces vary depending on the nature of. Liquid Surface Tension Effect.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Liquid Surface Tension Effect Cohesive forces between molecules cause the. Since these intermolecular forces vary depending on the nature of the. Attractive forces between molecules of different types are called adhesive forces. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount.. Liquid Surface Tension Effect.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation Liquid Surface Tension Effect Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Cohesive forces between molecules cause the. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. For the analysis. Liquid Surface Tension Effect.
From lessondbmetalepses.z21.web.core.windows.net
Does Temperature Affect Surface Tension Liquid Surface Tension Effect Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Attractive forces between molecules of different types are called adhesive forces. Surface tension \(\overline{\gamma}\) is defined to be the force f per. Liquid Surface Tension Effect.
From askfilo.com
\ 6 \rightarrow What is surface tension of liquids? Explain the effect o.. Liquid Surface Tension Effect Attractive forces between molecules of different types are called adhesive forces. For the analysis of the surface tension effects for. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Since these. Liquid Surface Tension Effect.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Liquid Surface Tension Effect In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. For the analysis of the surface tension effects for. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension is defined as the energy required to. Liquid Surface Tension Effect.
From pressbooks.uiowa.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Liquid Surface Tension Effect Attractive forces between molecules of different types are called adhesive forces. For the analysis of the surface tension effects for. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Since these intermolecular forces vary depending on the nature of the. In the absence of other forces, the surface tension makes a. Liquid Surface Tension Effect.
From chem.libretexts.org
11.3 Some Properties of Liquids Chemistry LibreTexts Liquid Surface Tension Effect In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. For the analysis of the surface tension effects for. Since these intermolecular forces vary depending on the nature of the. Attractive forces between molecules of different types are called adhesive forces. Surface tension is defined as. Liquid Surface Tension Effect.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Liquid Surface Tension Effect In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Attractive forces between molecules of. Liquid Surface Tension Effect.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Liquid Surface Tension Effect Cohesive forces between molecules cause the. Attractive forces between molecules of different types are called adhesive forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. For the analysis of the surface tension effects for. In the absence of other forces, the surface tension makes a liquid drop spherical. Liquid Surface Tension Effect.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Liquid Surface Tension Effect In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Since these intermolecular forces vary depending on the nature of the. Surface tension is defined as the energy required to increase the surface. Liquid Surface Tension Effect.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid Liquid Surface Tension Effect For the analysis of the surface tension effects for. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension. Liquid Surface Tension Effect.
From www.researchgate.net
Wetting behavior of a liquid droplet on rough surface (a) Young's Liquid Surface Tension Effect Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Attractive forces between molecules of different types are called adhesive forces. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its. Liquid Surface Tension Effect.
From www.slideserve.com
PPT Motivation for Studying Fluid Mechanics PowerPoint Presentation Liquid Surface Tension Effect Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Cohesive forces between molecules cause the. Attractive forces between molecules of different types are called adhesive forces. For the analysis of the surface tension effects for. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at. Liquid Surface Tension Effect.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Liquid Surface Tension Effect Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. For the analysis of the surface tension effects for. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Surface. Liquid Surface Tension Effect.
From www.researchgate.net
(PDF) Effects of liquid surface tension on gas capillaries and Liquid Surface Tension Effect In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Cohesive forces between molecules cause the. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension is the energy, or work, required to increase the surface. Liquid Surface Tension Effect.
From sophisticatedthoughtscom.wordpress.com
The Science Behind Bubbles (1/3) Sophisticated Thoughts Liquid Surface Tension Effect Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Since these intermolecular forces vary depending on the nature of the. Surface tension is proportional to the strength of the cohesive force, which varies with the type of. Liquid Surface Tension Effect.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Liquid Surface Tension Effect Since these intermolecular forces vary depending on the nature of the. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. For the analysis of the surface tension effects for. Surface tension is the energy, or work, required to. Liquid Surface Tension Effect.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Liquid Surface Tension Effect Attractive forces between molecules of different types are called adhesive forces. Since these intermolecular forces vary depending on the nature of the. For the analysis of the surface tension effects for. Cohesive forces between molecules cause the. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension is defined as the energy required to increase. Liquid Surface Tension Effect.
From www.youtube.com
What is Effect Of Temperature on surface tension Properties of Liquid Surface Tension Effect Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Attractive forces between molecules of different types are called adhesive forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is defined as the energy required to increase the. Liquid Surface Tension Effect.
From www.greenkidcrafts.com
Water Surface Tension Experiment for Kids Green Kid Crafts Liquid Surface Tension Effect Attractive forces between molecules of different types are called adhesive forces. For the analysis of the surface tension effects for. Cohesive forces between molecules cause the. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at. Liquid Surface Tension Effect.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Liquid Surface Tension Effect Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Since these intermolecular forces vary. Liquid Surface Tension Effect.