Titration Concentration Equation . In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. this can be worked out using the following equation: titration is a method to determine the unknown concentration of a specific substance. C a is the analyte. [7] c a = (c t × v t × m)/v a. this page titled 21.18: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. Concentration of unknown solution = (volume of known. the amount of added titrant is determined from its concentration and volume: The concentration of analyte may then be calculated using the following formula: a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
from general.chemistrysteps.com
this page titled 21.18: this can be worked out using the following equation: the amount of added titrant is determined from its concentration and volume: Concentration of unknown solution = (volume of known. [7] c a = (c t × v t × m)/v a. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The concentration of analyte may then be calculated using the following formula: titration is a method to determine the unknown concentration of a specific substance. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. C a is the analyte.
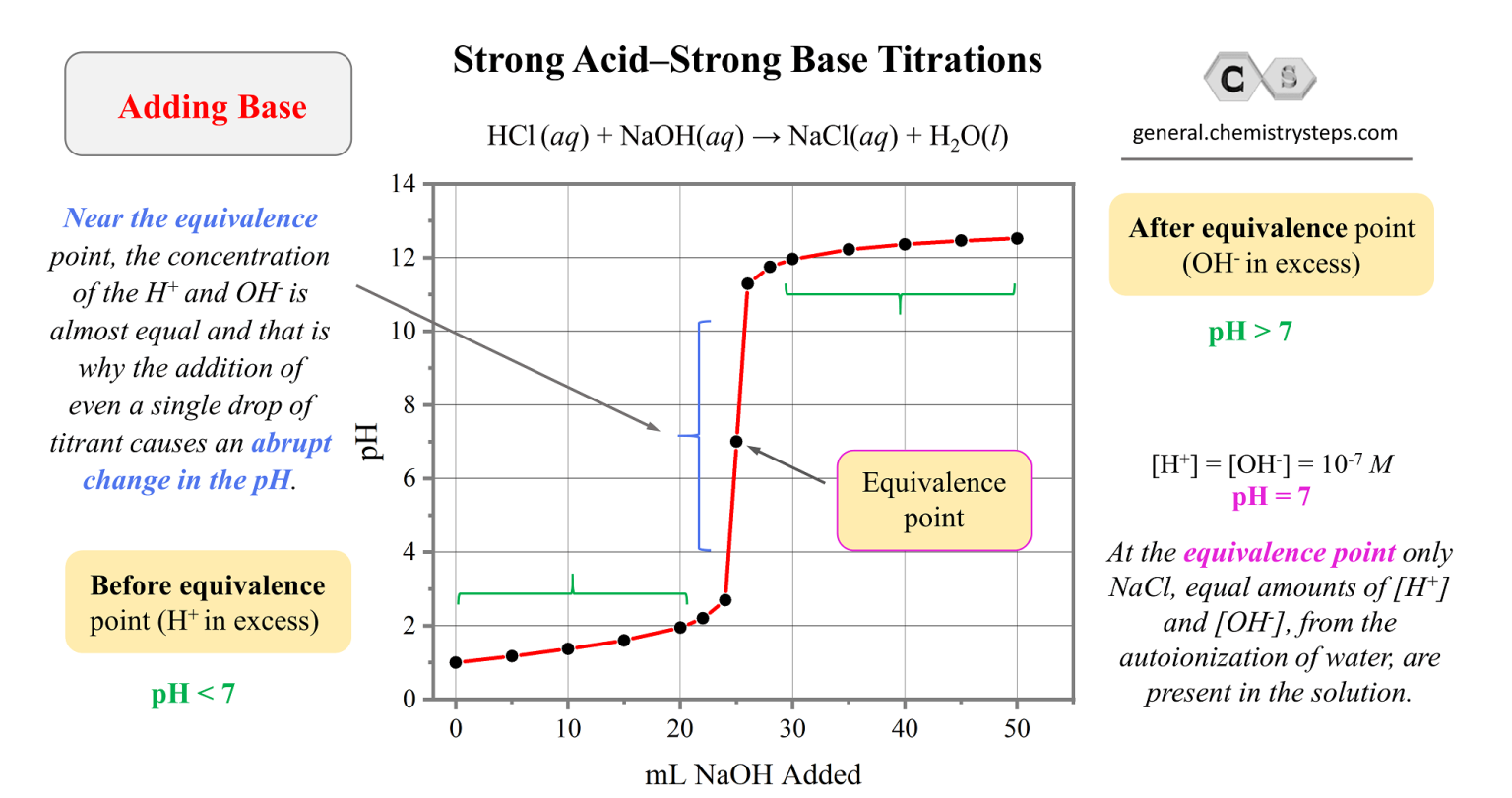
Strong AcidStrong Base Titrations Chemistry Steps
Titration Concentration Equation titration is a method to determine the unknown concentration of a specific substance. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. titration is a method to determine the unknown concentration of a specific substance. The concentration of analyte may then be calculated using the following formula: C a is the analyte. [7] c a = (c t × v t × m)/v a. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. this page titled 21.18: Concentration of unknown solution = (volume of known. this can be worked out using the following equation: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. the amount of added titrant is determined from its concentration and volume:
From dxopqqjgm.blob.core.windows.net
Titration Concentration Formula at Stephen Hansen blog Titration Concentration Equation In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. C a is the analyte. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Concentration of unknown solution = (volume of known. N (mol) = c (mol /l) * v (l) and the amount. Titration Concentration Equation.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Concentration Equation Concentration of unknown solution = (volume of known. The concentration of analyte may then be calculated using the following formula: a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. titration is a method. Titration Concentration Equation.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Concentration Equation a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The concentration of analyte may then be calculated using the following formula: C a is the analyte. this can be worked out using the following equation: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide. Titration Concentration Equation.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Concentration Equation titration is a method to determine the unknown concentration of a specific substance. [7] c a = (c t × v t × m)/v a. Concentration of unknown solution = (volume of known. C a is the analyte. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Titration Concentration Equation.
From exommzgkk.blob.core.windows.net
Titration Concentration Of Solution at Tony Pelletier blog Titration Concentration Equation this page titled 21.18: The concentration of analyte may then be calculated using the following formula: this can be worked out using the following equation: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. Titration Concentration Equation.
From exommzgkk.blob.core.windows.net
Titration Concentration Of Solution at Tony Pelletier blog Titration Concentration Equation [7] c a = (c t × v t × m)/v a. the amount of added titrant is determined from its concentration and volume: this page titled 21.18: Concentration of unknown solution = (volume of known. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In. Titration Concentration Equation.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Concentration Equation Concentration of unknown solution = (volume of known. [7] c a = (c t × v t × m)/v a. this can be worked out using the following equation: titration is a method to determine the unknown concentration of a specific substance. the amount of added titrant is determined from its concentration and volume: In a titration,. Titration Concentration Equation.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Concentration Equation [7] c a = (c t × v t × m)/v a. this page titled 21.18: titration is a method to determine the unknown concentration of a specific substance. Concentration of unknown solution = (volume of known. this can be worked out using the following equation: C a is the analyte. In a titration, 25.0 cm 3. Titration Concentration Equation.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube Titration Concentration Equation [7] c a = (c t × v t × m)/v a. the amount of added titrant is determined from its concentration and volume: this can be worked out using the following equation: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. N (mol) = c (mol /l) * v (l) and the. Titration Concentration Equation.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Titration Concentration Equation this page titled 21.18: this can be worked out using the following equation: titration is a method to determine the unknown concentration of a specific substance. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. Concentration of unknown solution = (volume of known. [7] c a = (c t × v t. Titration Concentration Equation.
From www.vrogue.co
Titration Concentration Calculations Complete Lesson vrogue.co Titration Concentration Equation a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Concentration of unknown solution = (volume of known. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. C a is the analyte. titration is a method to. Titration Concentration Equation.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution Titration Concentration Equation this can be worked out using the following equation: Concentration of unknown solution = (volume of known. the amount of added titrant is determined from its concentration and volume: [7] c a = (c t × v t × m)/v a. this page titled 21.18: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide. Titration Concentration Equation.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Concentration Equation the amount of added titrant is determined from its concentration and volume: this page titled 21.18: The concentration of analyte may then be calculated using the following formula: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. this can be worked out using the following equation: [7] c a = (c t. Titration Concentration Equation.
From dxopqqjgm.blob.core.windows.net
Titration Concentration Formula at Stephen Hansen blog Titration Concentration Equation C a is the analyte. the amount of added titrant is determined from its concentration and volume: titration is a method to determine the unknown concentration of a specific substance. The concentration of analyte may then be calculated using the following formula: Concentration of unknown solution = (volume of known. [7] c a = (c t × v. Titration Concentration Equation.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration Concentration Equation Concentration of unknown solution = (volume of known. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. [7] c a = (c t × v t × m)/v a. C a is the analyte. the amount of added titrant is determined from its concentration and volume: this page titled 21.18: this can. Titration Concentration Equation.
From chemistry.stackexchange.com
equilibrium How to calculate the dissociation constant of a weak acid Titration Concentration Equation C a is the analyte. Concentration of unknown solution = (volume of known. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. The concentration of analyte may then be calculated using the following formula: . Titration Concentration Equation.
From exommzgkk.blob.core.windows.net
Titration Concentration Of Solution at Tony Pelletier blog Titration Concentration Equation N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. The concentration of analyte may then be calculated using the following formula: this page titled 21.18: the amount of added titrant is determined from its concentration and volume: [7] c a = (c t × v t ×. Titration Concentration Equation.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Concentration Equation C a is the analyte. titration is a method to determine the unknown concentration of a specific substance. The concentration of analyte may then be calculated using the following formula: the amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be. Titration Concentration Equation.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration Concentration Equation this page titled 21.18: this can be worked out using the following equation: Concentration of unknown solution = (volume of known. [7] c a = (c t × v t × m)/v a. The concentration of analyte may then be calculated using the following formula: the amount of added titrant is determined from its concentration and volume:. Titration Concentration Equation.
From www.youtube.com
Titrations of Polyprotic Acids YouTube Titration Concentration Equation a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is a method to determine the unknown concentration of a specific substance. The concentration of analyte may then be calculated using the following formula: [7] c a = (c t × v t × m)/v a. . Titration Concentration Equation.
From dxopqqjgm.blob.core.windows.net
Titration Concentration Formula at Stephen Hansen blog Titration Concentration Equation The concentration of analyte may then be calculated using the following formula: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. titration is a method to determine the unknown concentration of a specific substance. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is.. Titration Concentration Equation.
From www.numerade.com
SOLVED Part III Titration Standardization of NaOH solution (finding Titration Concentration Equation C a is the analyte. The concentration of analyte may then be calculated using the following formula: titration is a method to determine the unknown concentration of a specific substance. [7] c a = (c t × v t × m)/v a. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. Titration Concentration Equation.
From morioh.com
Acid Base Titration Curves PH Calculations Titration Concentration Equation titration is a method to determine the unknown concentration of a specific substance. The concentration of analyte may then be calculated using the following formula: [7] c a = (c t × v t × m)/v a. this page titled 21.18: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. C a is. Titration Concentration Equation.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Concentration Equation titration is a method to determine the unknown concentration of a specific substance. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Concentration of unknown solution = (volume of known. The concentration of analyte may then be calculated using the following formula: the amount of added. Titration Concentration Equation.
From www.youtube.com
How to Calculate Concentration using Titration Example in Chemistry Titration Concentration Equation this page titled 21.18: a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The concentration of analyte may then be calculated using the following formula: Concentration of unknown solution = (volume of known. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is.. Titration Concentration Equation.
From www.youtube.com
GCSE Chemistry Titration calculations worked examples YouTube Titration Concentration Equation this page titled 21.18: a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. [7] c a = (c t × v t × m)/v a. the amount of added titrant is determined from its concentration and volume: titration is a method to determine the unknown. Titration Concentration Equation.
From www.youtube.com
Titration Calculations GCSE Science grade 7, 8 and 9 Booster Titration Concentration Equation the amount of added titrant is determined from its concentration and volume: C a is the analyte. The concentration of analyte may then be calculated using the following formula: this can be worked out using the following equation: Concentration of unknown solution = (volume of known. a titration is a laboratory technique used to precisely measure molar. Titration Concentration Equation.
From www.savemyexams.com
Titration Calculations Edexcel GCSE Chemistry Revision Notes 2018 Titration Concentration Equation titration is a method to determine the unknown concentration of a specific substance. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. N (mol) = c (mol /l) * v (l) and the. Titration Concentration Equation.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Titration Concentration Equation this can be worked out using the following equation: Concentration of unknown solution = (volume of known. the amount of added titrant is determined from its concentration and volume: titration is a method to determine the unknown concentration of a specific substance. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. . Titration Concentration Equation.
From www.youtube.com
Titration Calculations YouTube Titration Concentration Equation a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. The concentration of analyte may then be calculated using the following formula: titration is a method to determine. Titration Concentration Equation.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Titration Concentration Equation Concentration of unknown solution = (volume of known. this page titled 21.18: a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. this can be worked out using the following equation: [7] c a = (c t × v t × m)/v a. the amount of. Titration Concentration Equation.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Concentration Equation the amount of added titrant is determined from its concentration and volume: a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is a method to determine the unknown concentration of a specific substance. this can be worked out using the following equation: C a. Titration Concentration Equation.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Concentration Equation a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is a method to determine the unknown concentration of a specific substance. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. N (mol) = c (mol /l) * v (l) and the. Titration Concentration Equation.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Concentration Equation N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. Concentration of unknown solution = (volume of known. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. Titration Concentration Equation.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Concentration Equation C a is the analyte. titration is a method to determine the unknown concentration of a specific substance. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Titration Concentration Equation.