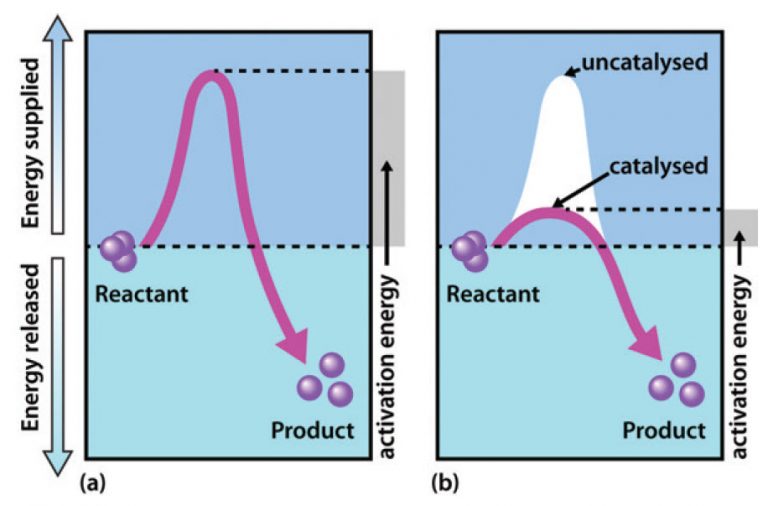
Catalysts In Reaction Mechanisms . In the first step, an enzyme molecule (e) and the substrate molecule. a homogeneous catalyst is present in the same phase as the reactants. a catalyst is a substance that increases the rate of a reaction without being consumed by it. It interacts with a reactant to form an intermediate. d37.2 catalysts and reaction mechanisms. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. 7.2 overview of catalytic mechanisms. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. A catalyst increases the rate of a reaction by altering the mechanism, allowing the. The effect of a catalyst is seen in its.
from wou.edu
catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a homogeneous catalyst is present in the same phase as the reactants. It interacts with a reactant to form an intermediate. d37.2 catalysts and reaction mechanisms. 7.2 overview of catalytic mechanisms. a catalyst is a substance that increases the rate of a reaction without being consumed by it. The effect of a catalyst is seen in its. In the first step, an enzyme molecule (e) and the substrate molecule. A catalyst increases the rate of a reaction by altering the mechanism, allowing the. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction.
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry
Catalysts In Reaction Mechanisms A catalyst increases the rate of a reaction by altering the mechanism, allowing the. The effect of a catalyst is seen in its. It interacts with a reactant to form an intermediate. 7.2 overview of catalytic mechanisms. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. In the first step, an enzyme molecule (e) and the substrate molecule. a homogeneous catalyst is present in the same phase as the reactants. d37.2 catalysts and reaction mechanisms. a catalyst is a substance that increases the rate of a reaction without being consumed by it. A catalyst increases the rate of a reaction by altering the mechanism, allowing the.
From www.youtube.com
Reaction Mechanisms Rate law practice and catalysts YouTube Catalysts In Reaction Mechanisms In the first step, an enzyme molecule (e) and the substrate molecule. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. The effect of a catalyst is seen in its. d37.2 catalysts and reaction mechanisms. It interacts with a reactant to form an intermediate.. Catalysts In Reaction Mechanisms.
From www.masterorganicchemistry.com
The 4 Major Classes of Reactions in Org 1 Master Organic Chemistry Catalysts In Reaction Mechanisms The effect of a catalyst is seen in its. It interacts with a reactant to form an intermediate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net. Catalysts In Reaction Mechanisms.
From www.masterorganicchemistry.com
Reaction Maps Now Available Master Organic Chemistry Catalysts In Reaction Mechanisms The effect of a catalyst is seen in its. It interacts with a reactant to form an intermediate. In the first step, an enzyme molecule (e) and the substrate molecule. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. A catalyst increases the rate of. Catalysts In Reaction Mechanisms.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Catalysts In Reaction Mechanisms It interacts with a reactant to form an intermediate. The effect of a catalyst is seen in its. A catalyst increases the rate of a reaction by altering the mechanism, allowing the. a homogeneous catalyst is present in the same phase as the reactants. In the first step, an enzyme molecule (e) and the substrate molecule. 7.2 overview. Catalysts In Reaction Mechanisms.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalysts In Reaction Mechanisms catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It interacts with a reactant to form an intermediate. a catalyst is a substance that increases the rate of a reaction without being consumed by it. The effect of a catalyst is seen in its. d37.2 catalysts and reaction. Catalysts In Reaction Mechanisms.
From www.slideserve.com
PPT 23.5 Features of homogeneous catalysis PowerPoint Presentation Catalysts In Reaction Mechanisms a homogeneous catalyst is present in the same phase as the reactants. 7.2 overview of catalytic mechanisms. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. d37.2 catalysts and reaction mechanisms. It interacts with a reactant to form an intermediate. catalysts. Catalysts In Reaction Mechanisms.
From www.slideserve.com
PPT Surfactants as Catalysts for Organic Reactions in Water Catalysts In Reaction Mechanisms The effect of a catalyst is seen in its. d37.2 catalysts and reaction mechanisms. A catalyst increases the rate of a reaction by altering the mechanism, allowing the. a homogeneous catalyst is present in the same phase as the reactants. 7.2 overview of catalytic mechanisms. a catalyst is a substance that increases the rate of a. Catalysts In Reaction Mechanisms.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalysts In Reaction Mechanisms The effect of a catalyst is seen in its. 7.2 overview of catalytic mechanisms. It interacts with a reactant to form an intermediate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a homogeneous catalyst is present in the same phase as the reactants. a catalyst is. Catalysts In Reaction Mechanisms.
From biologyease.com
Mechanism of Enzyme Catalysis Biology Ease Catalysts In Reaction Mechanisms catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a homogeneous catalyst is present in the same phase as the reactants. d37.2 catalysts and reaction mechanisms. 7.2 overview of catalytic mechanisms. The effect of a catalyst is seen in its. It interacts with a reactant to form. Catalysts In Reaction Mechanisms.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalysts In Reaction Mechanisms a catalyst is a substance that increases the rate of a reaction without being consumed by it. a homogeneous catalyst is present in the same phase as the reactants. 7.2 overview of catalytic mechanisms. The effect of a catalyst is seen in its. A catalyst increases the rate of a reaction by altering the mechanism, allowing the.. Catalysts In Reaction Mechanisms.
From www.masterorganicchemistry.com
Olefin Metathesis Master Organic Chemistry Catalysts In Reaction Mechanisms catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst is a substance that increases the rate of a reaction without being consumed by it. d37.2 catalysts and reaction mechanisms. The effect of a catalyst is seen in its. In the first step, an enzyme molecule (e). Catalysts In Reaction Mechanisms.
From www.youtube.com
01.10 Base Catalysis and Summary YouTube Catalysts In Reaction Mechanisms A catalyst increases the rate of a reaction by altering the mechanism, allowing the. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. The effect of a catalyst is seen in its. In the first step, an enzyme molecule (e) and the substrate molecule. catalysts are substances that speed. Catalysts In Reaction Mechanisms.
From www.slideserve.com
PPT 23.5 Features of homogeneous catalysis PowerPoint Presentation Catalysts In Reaction Mechanisms d37.2 catalysts and reaction mechanisms. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. a catalyst is a substance that increases the rate of a reaction without being consumed by it. It interacts with a reactant to form an intermediate. The effect of. Catalysts In Reaction Mechanisms.
From chem.libretexts.org
Chapter 14.8 Catalysis Chemistry LibreTexts Catalysts In Reaction Mechanisms d37.2 catalysts and reaction mechanisms. 7.2 overview of catalytic mechanisms. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. a catalyst is a. Catalysts In Reaction Mechanisms.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalysts In Reaction Mechanisms catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. It interacts with a reactant to form an intermediate. a homogeneous catalyst is present in the same phase as the reactants. catalysts affect the rate of a chemical reaction by altering its mechanism to. Catalysts In Reaction Mechanisms.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalysts In Reaction Mechanisms In the first step, an enzyme molecule (e) and the substrate molecule. a catalyst is a substance that increases the rate of a reaction without being consumed by it. The effect of a catalyst is seen in its. It interacts with a reactant to form an intermediate. catalysts are substances that speed up a reaction but which are. Catalysts In Reaction Mechanisms.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalysts In Reaction Mechanisms catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst is a substance that increases the rate of a reaction without being consumed by it. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net. Catalysts In Reaction Mechanisms.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalysts In Reaction Mechanisms 7.2 overview of catalytic mechanisms. a homogeneous catalyst is present in the same phase as the reactants. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower. Catalysts In Reaction Mechanisms.
From www.youtube.com
01.03 Mechanisms of Catalysis YouTube Catalysts In Reaction Mechanisms A catalyst increases the rate of a reaction by altering the mechanism, allowing the. a homogeneous catalyst is present in the same phase as the reactants. The effect of a catalyst is seen in its. 7.2 overview of catalytic mechanisms. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation. Catalysts In Reaction Mechanisms.
From www.researchgate.net
Catalysis reaction Reaction of Cu(i)Lcatalysed Catalysts In Reaction Mechanisms The effect of a catalyst is seen in its. It interacts with a reactant to form an intermediate. In the first step, an enzyme molecule (e) and the substrate molecule. 7.2 overview of catalytic mechanisms. a catalyst is a substance that increases the rate of a reaction without being consumed by it. A catalyst increases the rate of. Catalysts In Reaction Mechanisms.
From bianca-well-hooper.blogspot.com
Example of Combination Reaction in Which Catalyst Is Used Catalysts In Reaction Mechanisms It interacts with a reactant to form an intermediate. The effect of a catalyst is seen in its. A catalyst increases the rate of a reaction by altering the mechanism, allowing the. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. d37.2 catalysts and reaction mechanisms. a homogeneous. Catalysts In Reaction Mechanisms.
From biotechnologymcq.com
Enzyme V(II) Catalytic Mechanisms II Metal Ion catalysis; Catalysis by Catalysts In Reaction Mechanisms A catalyst increases the rate of a reaction by altering the mechanism, allowing the. The effect of a catalyst is seen in its. d37.2 catalysts and reaction mechanisms. It interacts with a reactant to form an intermediate. a homogeneous catalyst is present in the same phase as the reactants. a catalyst is a substance that increases the. Catalysts In Reaction Mechanisms.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysts In Reaction Mechanisms d37.2 catalysts and reaction mechanisms. It interacts with a reactant to form an intermediate. a homogeneous catalyst is present in the same phase as the reactants. The effect of a catalyst is seen in its. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst increases the. Catalysts In Reaction Mechanisms.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalysts In Reaction Mechanisms d37.2 catalysts and reaction mechanisms. a catalyst is a substance that increases the rate of a reaction without being consumed by it. The effect of a catalyst is seen in its. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a homogeneous catalyst is present in the. Catalysts In Reaction Mechanisms.
From www.youtube.com
01.09 General Acid Catalysis YouTube Catalysts In Reaction Mechanisms a homogeneous catalyst is present in the same phase as the reactants. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. 7.2 overview of catalytic mechanisms. It interacts with a reactant to form an intermediate. In the first step, an enzyme molecule (e) and the substrate molecule. . Catalysts In Reaction Mechanisms.
From www.researchgate.net
Oxidative and reductive quenching cycles within photoredox catalysis Catalysts In Reaction Mechanisms a homogeneous catalyst is present in the same phase as the reactants. In the first step, an enzyme molecule (e) and the substrate molecule. a catalyst is a substance that increases the rate of a reaction without being consumed by it. 7.2 overview of catalytic mechanisms. The effect of a catalyst is seen in its. It interacts. Catalysts In Reaction Mechanisms.
From www.researchgate.net
Mechanisms of ester hydrolysis under acid or base catalysis. Download Catalysts In Reaction Mechanisms The effect of a catalyst is seen in its. It interacts with a reactant to form an intermediate. a homogeneous catalyst is present in the same phase as the reactants. a catalyst is a substance that increases the rate of a reaction without being consumed by it. In the first step, an enzyme molecule (e) and the substrate. Catalysts In Reaction Mechanisms.
From www.masterorganicchemistry.com
Acid Catalysis of Organic Reactions Why It Works Catalysts In Reaction Mechanisms It interacts with a reactant to form an intermediate. 7.2 overview of catalytic mechanisms. d37.2 catalysts and reaction mechanisms. a homogeneous catalyst is present in the same phase as the reactants. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst is a substance that. Catalysts In Reaction Mechanisms.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysts In Reaction Mechanisms a catalyst is a substance that increases the rate of a reaction without being consumed by it. In the first step, an enzyme molecule (e) and the substrate molecule. d37.2 catalysts and reaction mechanisms. a homogeneous catalyst is present in the same phase as the reactants. A catalyst increases the rate of a reaction by altering the. Catalysts In Reaction Mechanisms.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalysts In Reaction Mechanisms A catalyst increases the rate of a reaction by altering the mechanism, allowing the. a catalyst is a substance that increases the rate of a reaction without being consumed by it. It interacts with a reactant to form an intermediate. The effect of a catalyst is seen in its. In the first step, an enzyme molecule (e) and the. Catalysts In Reaction Mechanisms.
From www.slideserve.com
PPT Reaction mechanisms and catalysts PowerPoint Presentation, free Catalysts In Reaction Mechanisms A catalyst increases the rate of a reaction by altering the mechanism, allowing the. It interacts with a reactant to form an intermediate. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. catalysts affect the rate of a chemical reaction by altering its mechanism. Catalysts In Reaction Mechanisms.
From chem.libretexts.org
Chapter 13.8 Catalysis Chemistry LibreTexts Catalysts In Reaction Mechanisms The effect of a catalyst is seen in its. a homogeneous catalyst is present in the same phase as the reactants. catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. d37.2 catalysts and reaction mechanisms. catalysts affect the rate of a chemical. Catalysts In Reaction Mechanisms.
From www.researchgate.net
Generic mechanisms for N 2 reduction to NH 3 on heterogeneous catalysts Catalysts In Reaction Mechanisms It interacts with a reactant to form an intermediate. In the first step, an enzyme molecule (e) and the substrate molecule. The effect of a catalyst is seen in its. A catalyst increases the rate of a reaction by altering the mechanism, allowing the. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a. Catalysts In Reaction Mechanisms.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Catalysts In Reaction Mechanisms In the first step, an enzyme molecule (e) and the substrate molecule. a homogeneous catalyst is present in the same phase as the reactants. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It interacts with a reactant to form an intermediate. d37.2 catalysts and reaction mechanisms. The. Catalysts In Reaction Mechanisms.
From www.bnl.gov
Increasing the Efficiency of CO Catalytic Conversion BNL Newsroom Catalysts In Reaction Mechanisms catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. It interacts with a reactant to form an intermediate. A catalyst increases the rate of a reaction by altering the mechanism, allowing the. catalysts affect the rate of a chemical reaction by altering its mechanism. Catalysts In Reaction Mechanisms.