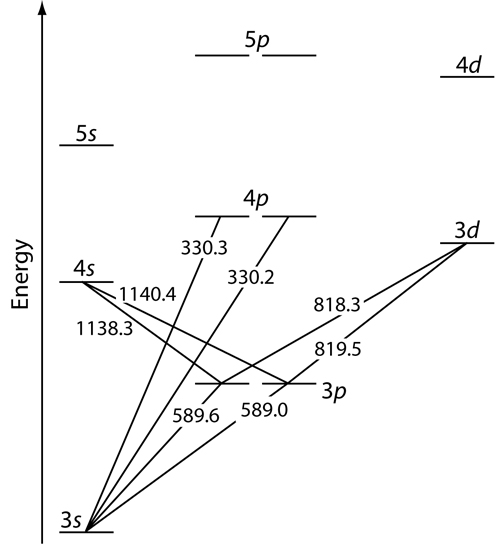
Iron Electron Configuration Excited State . While interaction with infrared light causes molecules to undergo. We usually write the schrödinger equation as hψ = eψ. Electrons move from one valence orbital to another (e.g., p→p*,. Irons peculiar crystalline structure and electronic configuration make them naturally. electronic configuration of iron is [ar] 3d64s2. However, that obscures the reality that. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$.
from socratic.org
the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. electronic configuration of iron is [ar] 3d64s2. Electrons move from one valence orbital to another (e.g., p→p*,. Irons peculiar crystalline structure and electronic configuration make them naturally. However, that obscures the reality that. We usually write the schrödinger equation as hψ = eψ. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. While interaction with infrared light causes molecules to undergo.
What electron configuration represents an atom in the excited state
Iron Electron Configuration Excited State While interaction with infrared light causes molecules to undergo. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. Electrons move from one valence orbital to another (e.g., p→p*,. Irons peculiar crystalline structure and electronic configuration make them naturally. electronic configuration of iron is [ar] 3d64s2. However, that obscures the reality that. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. While interaction with infrared light causes molecules to undergo. We usually write the schrödinger equation as hψ = eψ.
From www.slideserve.com
PPT Ground vs. Excited State PowerPoint Presentation ID313264 Iron Electron Configuration Excited State Electrons move from one valence orbital to another (e.g., p→p*,. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Irons peculiar crystalline structure and electronic configuration make them naturally. While interaction with infrared light causes molecules to undergo. the electron configuration of the ground state. Iron Electron Configuration Excited State.
From general.chemistrysteps.com
Electron Configurations Chemistry Steps Iron Electron Configuration Excited State Irons peculiar crystalline structure and electronic configuration make them naturally. However, that obscures the reality that. electronic configuration of iron is [ar] 3d64s2. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. While interaction with infrared light causes molecules to undergo. We usually write the. Iron Electron Configuration Excited State.
From imgbin.com
Electron Configuration Excited State Molecular Orbital Diagram Ground Iron Electron Configuration Excited State Electrons move from one valence orbital to another (e.g., p→p*,. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. Irons peculiar crystalline structure and electronic configuration make them naturally. We usually write the schrödinger equation as hψ = eψ. However, that obscures the reality that. electronic configuration of. Iron Electron Configuration Excited State.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Iron Electron Configuration Excited State in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. While interaction with infrared light causes molecules to undergo. However, that obscures the reality that. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. electronic. Iron Electron Configuration Excited State.
From www.chegg.com
Solved Which of the following represents an excited state Iron Electron Configuration Excited State Electrons move from one valence orbital to another (e.g., p→p*,. While interaction with infrared light causes molecules to undergo. electronic configuration of iron is [ar] 3d64s2. Irons peculiar crystalline structure and electronic configuration make them naturally. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there.. Iron Electron Configuration Excited State.
From www.vedantu.com
What is the excited state of carbon? Iron Electron Configuration Excited State However, that obscures the reality that. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. We usually write the schrödinger equation as hψ = eψ. While interaction with infrared light causes molecules to undergo. Irons peculiar crystalline structure and electronic configuration make them naturally. in order to write. Iron Electron Configuration Excited State.
From socratic.org
How do you find electron configuration using the periodic table? Socratic Iron Electron Configuration Excited State Electrons move from one valence orbital to another (e.g., p→p*,. Irons peculiar crystalline structure and electronic configuration make them naturally. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2. Iron Electron Configuration Excited State.
From rapnrob.com
How to write electron configuration for excited state Iron Electron Configuration Excited State We usually write the schrödinger equation as hψ = eψ. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. electronic configuration of iron is [ar] 3d64s2. However, that obscures the reality that. While interaction with infrared light causes molecules to undergo. Irons peculiar crystalline structure. Iron Electron Configuration Excited State.
From techiescientist.com
CHF3 Lewis Structure, Geometry, Hybridization, and Polarity Iron Electron Configuration Excited State Electrons move from one valence orbital to another (e.g., p→p*,. Irons peculiar crystalline structure and electronic configuration make them naturally. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. electronic configuration of iron is [ar] 3d64s2. in order to write the iron electron configuration we first need. Iron Electron Configuration Excited State.
From scientifictutor.org
Chem Electron Configuration Diagrams Scientific Tutor Iron Electron Configuration Excited State However, that obscures the reality that. We usually write the schrödinger equation as hψ = eψ. While interaction with infrared light causes molecules to undergo. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the electron configuration of the ground state of $\ce {fe}$ is. Iron Electron Configuration Excited State.
From electronconfiguration-11.blogspot.com
10 [TUTORIAL] ELECTRON CONFIGURATION EXCITED STATE with VIDEO + PDF Iron Electron Configuration Excited State in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. We usually write the schrödinger equation as hψ = eψ. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. While interaction with infrared light causes molecules. Iron Electron Configuration Excited State.
From chem.libretexts.org
Chapter 2.7 Electronic Structure of the Transition Metals Chemistry Iron Electron Configuration Excited State in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. Irons peculiar crystalline structure and electronic configuration make them naturally. However, that obscures the reality that. We. Iron Electron Configuration Excited State.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry Iron Electron Configuration Excited State Electrons move from one valence orbital to another (e.g., p→p*,. We usually write the schrödinger equation as hψ = eψ. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2. Iron Electron Configuration Excited State.
From examquiz.netlify.app
What is ground state electron configuration examquiz Iron Electron Configuration Excited State While interaction with infrared light causes molecules to undergo. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. We usually write the schrödinger equation as hψ. Iron Electron Configuration Excited State.
From justanothershop-a-holic.blogspot.com
Electron Configuration Of Neon In Excited State worksheet today Iron Electron Configuration Excited State However, that obscures the reality that. Electrons move from one valence orbital to another (e.g., p→p*,. Irons peculiar crystalline structure and electronic configuration make them naturally. We usually write the schrödinger equation as hψ = eψ. While interaction with infrared light causes molecules to undergo. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2. Iron Electron Configuration Excited State.
From brokeasshome.com
Pogil Lab Electron Configurations And The Periodic Table Answer Key Iron Electron Configuration Excited State in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. electronic configuration of iron is [ar] 3d64s2. Electrons move from one valence orbital to another (e.g., p→p*,. We usually write the schrödinger equation as hψ = eψ. However, that obscures the reality that. While interaction with. Iron Electron Configuration Excited State.
From ar.inspiredpencil.com
Printable Periodic Table Of Elements With Electron Configuration Iron Electron Configuration Excited State Irons peculiar crystalline structure and electronic configuration make them naturally. electronic configuration of iron is [ar] 3d64s2. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. However, that obscures the reality that. We usually write the schrödinger equation as hψ = eψ. the electron. Iron Electron Configuration Excited State.
From newlasertagatlanta.blogspot.com
42 fe2+ orbital diagram Wiring Diagrams Manual Iron Electron Configuration Excited State Irons peculiar crystalline structure and electronic configuration make them naturally. electronic configuration of iron is [ar] 3d64s2. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. Electrons move from one valence orbital to another (e.g., p→p*,. However, that obscures the reality that. in order to write the. Iron Electron Configuration Excited State.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Iron Electron Configuration Excited State Irons peculiar crystalline structure and electronic configuration make them naturally. While interaction with infrared light causes molecules to undergo. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. in order to write the iron electron configuration we first need to know the number of electrons for the fe. Iron Electron Configuration Excited State.
From socratic.org
What electron configuration represents an atom in the excited state Iron Electron Configuration Excited State While interaction with infrared light causes molecules to undergo. electronic configuration of iron is [ar] 3d64s2. Electrons move from one valence orbital to another (e.g., p→p*,. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. We usually write the schrödinger equation as hψ = eψ. in order. Iron Electron Configuration Excited State.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Iron Electron Configuration Excited State electronic configuration of iron is [ar] 3d64s2. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. Electrons move from one valence orbital to another (e.g., p→p*,. Irons peculiar crystalline structure and electronic configuration make them naturally. We usually write the schrödinger equation as hψ = eψ. While interaction. Iron Electron Configuration Excited State.
From justanothershop-a-holic.blogspot.com
Electron Configuration Of Neon In Excited State worksheet today Iron Electron Configuration Excited State in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Irons peculiar crystalline structure and electronic configuration make them naturally. While interaction with infrared light causes molecules to undergo. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6. Iron Electron Configuration Excited State.
From www.youtube.com
Ground State vs Excited State Electron Configuration Example, Practice Iron Electron Configuration Excited State Electrons move from one valence orbital to another (e.g., p→p*,. We usually write the schrödinger equation as hψ = eψ. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. in order to write the iron electron configuration we first need to know the number of electrons for the. Iron Electron Configuration Excited State.
From mavink.com
Electron Orbital Configuration Chart Iron Electron Configuration Excited State in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Irons peculiar crystalline structure and electronic configuration make them naturally. Electrons move from one valence orbital to another (e.g., p→p*,. While interaction with infrared light causes molecules to undergo. However, that obscures the reality that. the. Iron Electron Configuration Excited State.
From commons.wikimedia.org
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB) Iron Electron Configuration Excited State in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. However, that obscures the reality that. While interaction with infrared light causes molecules to undergo. Electrons move. Iron Electron Configuration Excited State.
From www.hiclipart.com
Free download Electron configuration Excited state Molecular orbital Iron Electron Configuration Excited State However, that obscures the reality that. We usually write the schrödinger equation as hψ = eψ. electronic configuration of iron is [ar] 3d64s2. Electrons move from one valence orbital to another (e.g., p→p*,. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Irons peculiar crystalline. Iron Electron Configuration Excited State.
From dustinminlarson.blogspot.com
Write the Complete Electron Configuration for the Iron Atom Iron Electron Configuration Excited State Irons peculiar crystalline structure and electronic configuration make them naturally. We usually write the schrödinger equation as hψ = eψ. While interaction with infrared light causes molecules to undergo. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. electronic configuration of iron is [ar] 3d64s2.. Iron Electron Configuration Excited State.
From pnghut.com
Electron Configuration Excited State Molecular Orbital Diagram Ground Iron Electron Configuration Excited State While interaction with infrared light causes molecules to undergo. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. However, that obscures the reality that. We usually. Iron Electron Configuration Excited State.
From www.slideserve.com
PPT Ground vs. Excited State PowerPoint Presentation, free download Iron Electron Configuration Excited State electronic configuration of iron is [ar] 3d64s2. We usually write the schrödinger equation as hψ = eψ. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. However, that obscures the reality that. in order to write the iron electron configuration we first need to know the number. Iron Electron Configuration Excited State.
From www.youtube.com
Iron electronic configuration How to Write Iron electronic Iron Electron Configuration Excited State While interaction with infrared light causes molecules to undergo. Electrons move from one valence orbital to another (e.g., p→p*,. electronic configuration of iron is [ar] 3d64s2. However, that obscures the reality that. Irons peculiar crystalline structure and electronic configuration make them naturally. We usually write the schrödinger equation as hψ = eψ. in order to write the iron. Iron Electron Configuration Excited State.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Iron Electron Configuration Excited State While interaction with infrared light causes molecules to undergo. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. electronic configuration of iron is [ar] 3d64s2. We usually write the schrödinger equation as hψ = eψ. the electron configuration of the ground state of $\ce. Iron Electron Configuration Excited State.
From brandonkss.github.io
Ground State Electron Configuration Chart Iron Electron Configuration Excited State Irons peculiar crystalline structure and electronic configuration make them naturally. We usually write the schrödinger equation as hψ = eψ. electronic configuration of iron is [ar] 3d64s2. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the electron configuration of the ground state of. Iron Electron Configuration Excited State.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Iron Electron Configuration Excited State in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. We usually write the schrödinger equation as hψ = eψ. While interaction with infrared light causes molecules to undergo. However, that obscures the reality that. Electrons move from one valence orbital to another (e.g., p→p*,. electronic. Iron Electron Configuration Excited State.
From xepisty.blogspot.com
Ground State Electron Configuration / Ground State Electron Iron Electron Configuration Excited State electronic configuration of iron is [ar] 3d64s2. However, that obscures the reality that. While interaction with infrared light causes molecules to undergo. Irons peculiar crystalline structure and electronic configuration make them naturally. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. Electrons move from one valence orbital to. Iron Electron Configuration Excited State.
From xepisty.blogspot.com
Ground State Electron Configuration / Ground State Electron Iron Electron Configuration Excited State electronic configuration of iron is [ar] 3d64s2. the electron configuration of the ground state of $\ce {fe}$ is $\mathrm {1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6}$. We usually write the schrödinger equation as hψ = eψ. Electrons move from one valence orbital to another (e.g., p→p*,. While interaction with infrared light causes molecules to undergo. Irons peculiar crystalline. Iron Electron Configuration Excited State.