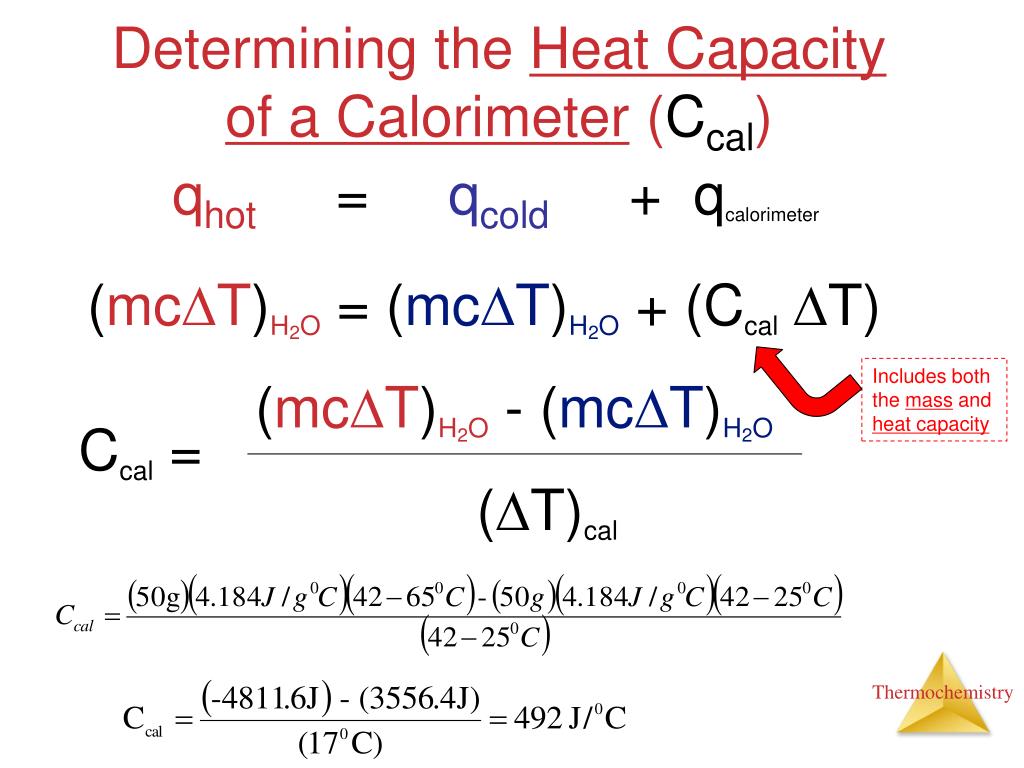
A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature . to determine the equilibrium temperature when 100 g of water at 40°c is poured into a calorimeter with a heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate and interpret heat and related properties. one of the major effects of heat transfer is temperature change: a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. a calorimeter of heat capacity 100j /k is at room temperature of 30∘c. Heating increases the temperature while cooling. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: 100 g of water at 40°c of specific. We are talking about the calories in the numerical. a calorimeter of heat capacity 100 j/k is at room temperature of 30∘c.100 g of water at 40∘c of specifie heat 4200. the calorimeter heat capacity is 1.23 j/k. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. where q is the heat generation rate of the cement (w/m 3), x, y, z represent three different axes in the concrete, t is the. to find the final temperature of the water in the calorimeter, we will use the principle of conservation of energy, which states that.
from www.slideserve.com
to find the final temperature of the water in the calorimeter, we will use the principle of conservation of energy, which states that. a calorimeter of heat capacity 100j /k is at room temperature of 30∘c. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. We are talking about the calories in the numerical. Calculate and interpret heat and related properties. Explain the technique of calorimetry. 100 g of water at 40°c of specific. a calorimeter of heat capacity 100 j/k is at room temperature of 30°c. a calorimeter of heat capacity `100 j//k` is at room temperature of `30^(@)c`.
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download
A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature Calorimetry is the measurement of heat flow. Given that the final temperature at thermal equilibrium is 26.2 °c, determine the. It can analyze the heat exchange between up to 3 objects. a calorimeter of heat capacity `100 j//k` is at room temperature of `30^(@)c`. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. a calorimeter of heat capacity 100j /k is at room temperature of 30∘c. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to. a calorimeter of heat capacity 100 j/k is at room temperature of 30°c. calculate the heat capacity of the calorimeter in j/°c. We are talking about the calories in the numerical. Heat energy flows from a substance that. Calorimetry is the measurement of heat flow. Heating increases the temperature while cooling. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. If the heat capacity of the.
From boisestate.pressbooks.pub
9.2 Calorimetry General Chemistry 1 & 2 A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. Heating increases the temperature while cooling. a calorimeter of heat capacity 100 j/k is at room temperature of 30â°c. Compare heat flow from hot to cold objects in an ideal. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.chegg.com
Solved Calculate Heat capacity of the calorimeter (J/ ºC) , A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature how do you measure calorimetry? to find the final temperature of the water in the calorimeter, we will use the principle of conservation of energy, which states that. the calorimetry calculator can help you solve complex calorimetry problems. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. I. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.youtube.com
Principle of Calorimetry YouTube A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature It can analyze the heat exchange between up to 3 objects. Calorimetry is the measurement of heat flow. one of the major effects of heat transfer is temperature change: to find the final temperature of the water in the calorimeter, we will use the principle of conservation of energy, which states that. 100 g of water at 40°c. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a container that prevents heat transfer in or out is called a calorimeter, and the. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From exouxbqmv.blob.core.windows.net
Calorimetry Heat Of Formation at Dolores Slama blog A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature a calorimeter of heat capacity `100 j//k` is at room temperature of `30^(@)c`. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. Calorimetry is the measurement of heat flow. a calorimeter of heat capacity 100j /k is at. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6133898 A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature Heat energy flows from a substance that. normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: We are talking about the calories in the numerical. how. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature `100g` of water at `40^(@)c` of. Given that the final temperature at thermal equilibrium is 26.2 °c, determine the. Heating increases the temperature while cooling. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: Explain the technique of calorimetry. where q is the heat generation rate of the cement (w/m 3), x, y, z. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature the calorimeter heat capacity is 1.23 j/k. I would like to introduce myself to everyone. to find the final temperature of the water in the calorimeter, we will use the principle of conservation of energy, which states that. one of the major effects of heat transfer is temperature change: It can analyze the heat exchange between up. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature the calorimeter heat capacity is 1.23 j/k. Explain the technique of calorimetry. a calorimeter of heat capacity 100 j/k is at room temperature of 30°c. to determine the equilibrium temperature when 100 g of water at 40°c is poured into a calorimeter with a heat. when 1.00 g of coal is burned in a bomb calorimeter,. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature `100g` of water at `40^(@)c` of. 100 g of water at 40â°c with specific heat. calculate the heat capacity of the calorimeter in j/°c. the calorimeter heat capacity is 1.23 j/k. a calorimeter of heat capacity 100 j/k is at room temperature of 30∘c.100 g of water at 40∘c of specifie heat 4200. to determine the. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature We are talking about the calories in the numerical. normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. to find the final temperature of the water in the calorimeter, we will use the principle of conservation of energy, which states. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.youtube.com
Heat of Reaction from a Calorimeter (Example) YouTube A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature the calorimeter heat capacity is 1.23 j/k. Calculate and interpret heat and related properties. normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. a calorimeter of heat capacity `100 j//k` is at room temperature of `30^(@)c`. Given that the. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. one of the major effects of heat transfer is temperature change: the calorimeter constant. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.doubtnut.com
A calorimeter of heat capacity 100 J//K is at room temperature of 30^( A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature to determine the equilibrium temperature when 100 g of water at 40°c is poured into a calorimeter with a heat. Given that the final temperature at thermal equilibrium is 26.2 °c, determine the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. normally it can be done by heating a piece. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.chegg.com
Chemistry Archive November 05, 2016 A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. calculate the heat capacity of the calorimeter in j/°c. a calorimeter of heat capacity `100 j//k` is at room temperature of `30^(@)c`. a calorimeter of heat capacity 100. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter Stepbystep A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature Calorimetry is the measurement of heat flow. normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. I would like to introduce myself to everyone. a calorimeter of heat capacity `100 j//k` is at room temperature of `30^(@)c`. a calorimeter. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.qwivy.com
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature 100g of water at 40∘c of specific heat 4200j /kg − k is. Calorimetry is the measurement of heat flow. calculate the heat capacity of the calorimeter in j/°c. I would like to introduce myself to everyone. a calorimeter of heat capacity 100j /k is at room temperature of 30∘c. If the heat capacity of the. 100 g. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature a calorimeter of heat capacity 100 j/k is at room temperature of 30°c. Given that the final temperature at thermal equilibrium is 26.2 °c, determine the. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: a calorimeter of heat capacity 100 j/k is at room temperature of 30°c. Heating increases the temperature while. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature I would like to introduce myself to everyone. Calculate and interpret heat and related properties. a calorimeter of heat capacity 100 j/k is at room temperature of 30â°c. how do you measure calorimetry? one of the major effects of heat transfer is temperature change: We are talking about the calories in the numerical. a calorimeter of. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.toppr.com
A calorimeter of mass 50 g and specific heat capacity 0.42 J g^1°C^1 A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature Explain the technique of calorimetry. We are talking about the calories in the numerical. where q is the heat generation rate of the cement (w/m 3), x, y, z represent three different axes in the concrete, t is the. calculate the heat capacity of the calorimeter in j/°c. normally it can be done by heating a piece. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature Calorimetry is the measurement of heat flow. the calorimetry calculator can help you solve complex calorimetry problems. where q is the heat generation rate of the cement (w/m 3), x, y, z represent three different axes in the concrete, t is the. to determine the equilibrium temperature when 100 g of water at 40°c is poured into. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.chegg.com
Solved Calculations I. Heat Capacity of the Calorimeter The A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature a calorimeter of heat capacity 100 j/k is at room temperature of 30°c. a calorimeter of heat capacity 100 j/k is at room temperature of 30â°c. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. `100g` of water at `40^(@)c` of. the calorimetry calculator can help you solve. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature a calorimeter of heat capacity 100 j/k is at room temperature of 30∘c.100 g of water at 40∘c of specifie heat 4200. Explain the technique of calorimetry. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: apply the first law of thermodynamics to calorimetry ; I would like to introduce myself to everyone.. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.toppr.com
A calorimeter of heat capacity 100 J/K is room temperature of 30°C. 100 A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature I would like to introduce myself to everyone. the calorimetry calculator can help you solve complex calorimetry problems. one of the major effects of heat transfer is temperature change: the calorimeter heat capacity is 1.23 j/k. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. 100g of water. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.chegg.com
Solved A bomb calorimeter has a heat capacity of 2.47 kJ/K. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: the calorimetry calculator can help you solve complex calorimetry problems. We are talking about the calories in the numerical. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is the measurement of heat flow. a calorimeter. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.toppr.com
A calorimeter of heat capacity 100 J/K is room temperature of 30°C. 100 A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: It can analyze the heat exchange between up to 3 objects. 100 g of water at 40â°c with specific heat. how do you measure calorimetry? Explain the technique of calorimetry. Calorimetry is the measurement of heat flow. when 1.00 g of coal is burned. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From users.highland.edu
Calorimetry A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature where q is the heat generation rate of the cement (w/m 3), x, y, z represent three different axes in the concrete, t is the. If the heat capacity of the. I would like to introduce myself to everyone. the calorimeter heat capacity is 1.23 j/k. the calorimeter constant is usually presented in units of joules per. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.toppr.com
In a calorimeter of water equivalent 20g ,water of mass 1.1 kg is taken A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. where q is the heat generation rate of the cement (w/m 3), x, y, z represent three different axes in the concrete, t is the. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c.. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.toppr.com
A copper calorimeter of a mass 300 g contains 500 g of water at a A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature `100g` of water at `40^(@)c` of. the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. Heat energy flows from a substance that. how do you measure calorimetry? to determine the equilibrium temperature when 100 g of water at 40°c is poured into a calorimeter with. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.toppr.com
partly Hence temperature of mixture will remain 100°C. stration 14 A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature If the heat capacity of the. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. a calorimeter of heat capacity 100 j/k is at room temperature of 30â°c. to find the final temperature of the water in the calorimeter, we will use the principle. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From frankmccomb.info
Which Statement Describes How a Basic Coffee Cup Calorimeter Works? A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to. a calorimeter of heat capacity 100 j/k is at room temperature of 30â°c. a calorimeter of heat capacity 100j /k is at room temperature of 30∘c. Calorimetry is the measurement of heat flow. Explain the technique of calorimetry. . A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.numerade.com
SOLVED When a solid dissolves in water; heat may be evolved or A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature 100g of water at 40∘c of specific heat 4200j /kg − k is. how do you measure calorimetry? apply the first law of thermodynamics to calorimetry ; to determine the equilibrium temperature when 100 g of water at 40°c is poured into a calorimeter with a heat. Explain the technique of calorimetry. `100g` of water at `40^(@)c`. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.youtube.com
050 Calorimetry YouTube A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature It can analyze the heat exchange between up to 3 objects. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to. a calorimeter of heat capacity 100j /k is at room temperature of 30∘c. the calorimeter constant is usually presented in units of joules per degree celsius (j/°c) or. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature a calorimeter of heat capacity 100 j/k is at room temperature of 30∘c.100 g of water at 40∘c of specifie heat 4200. We are talking about the calories in the numerical. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature one of the major effects of heat transfer is temperature change: I would like to introduce myself to everyone. 100g of water at 40∘c of specific heat 4200j /kg − k is. to find the final temperature of the water in the calorimeter, we will use the principle of conservation of energy, which states that. how do. A Calorimeter Of Heat Capacity 100 J/K Is At Room Temperature.