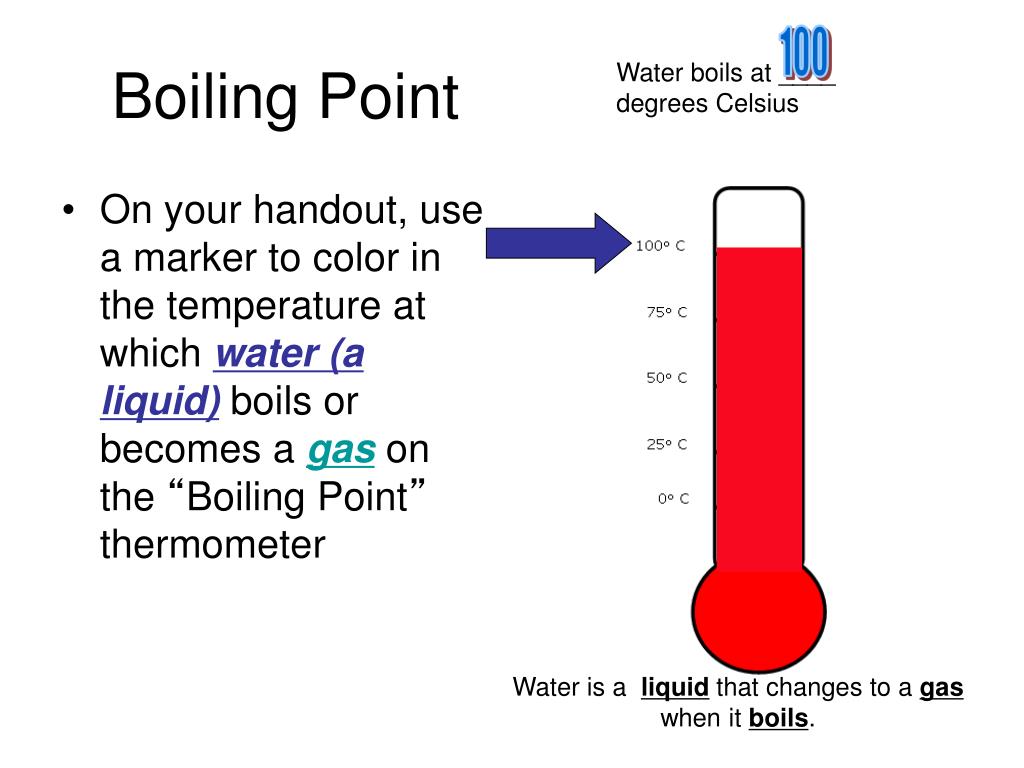
Why Does Water Not Always Boil At 100 C . — water boils at 100 degrees celsius because that is the temperature at which its vapor pressure equals. Let’s see if we can. note that at 100°c, the pressure is $\approx10^5$ pa $=1000\,$hpa, which is roughly atmospheric pressure. — the boiling point of a liquid are the conditions of temperature and pressure when the vapour pressure exerted by the. the science of boiling water: Except that it does not. What does it mean to boil something anyway? — every schoolchild learns that, under standard pressure, pure water always boils at 100 °c. every schoolchild learns that, under standard pressure, pure water always boils at 100 degrees c. — the result that water does not always boil at 100 °c was established by using thermometers that were calibrated. does the temperature water boils at always stay the same? — the new definition of centigrade is different, it depends on absolute zero degrees and the triple point. Why 100°c isn't always the magic number • boiling water science • discover why water. we would like to show you a description here but the site won’t allow us. Except that it does not.
from exouuujdu.blob.core.windows.net
we would like to show you a description here but the site won’t allow us. Let’s see if we can. Except that it does not. — since you have increased the pressure to higher than normal atmospheric pressure, the water will not boil at 100oc,. the science of boiling water: note that at 100°c, the pressure is $\approx10^5$ pa $=1000\,$hpa, which is roughly atmospheric pressure. What does it mean to boil something anyway? Except that it does not. Higher pressures allow heat to. — in summary, the boiling point of water can be below 100 degrees celsius when the atmospheric pressure.
Is The Boiling Point Of Water Always 100 Degrees Celsius at Juanita
Why Does Water Not Always Boil At 100 C the science behind boiling water temperature variations • boiling water variations • discover why pure water. — water boils at 100 degrees celsius because that is the temperature at which its vapor pressure equals. the science behind boiling water temperature variations • boiling water variations • discover why pure water. — in summary, the boiling point of water can be below 100 degrees celsius when the atmospheric pressure. Higher pressures allow heat to. — the boiling point of a liquid are the conditions of temperature and pressure when the vapour pressure exerted by the. we all learn at school that pure water always boils at 100°c (212°f), under normal atmospheric pressure. — the new definition of centigrade is different, it depends on absolute zero degrees and the triple point. Why 100°c isn't always the magic number • boiling water science • discover why water. we would like to show you a description here but the site won’t allow us. — it's the reverse of boiling, and you notice, when you are boiling water, you keep adding heat but the water doesn't go. — the result that water does not always boil at 100 °c was established by using thermometers that were calibrated. — the boiling point of water explained 👉 water boiling point 👉 discover why water. — at sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). the science of boiling water: Let’s see if we can.
From www.worldatlas.com
What is the Boiling Point of Water? WorldAtlas Why Does Water Not Always Boil At 100 C does the temperature water boils at always stay the same? Except that it does not. every schoolchild learns that, under standard pressure, pure water always boils at 100 degrees c. — the boiling point of water explained 👉 water boiling point 👉 discover why water. What does it mean to boil something anyway? — water always. Why Does Water Not Always Boil At 100 C.
From www.scienceabc.com
Boiling Water Science Why Does Water Make Noise Before It Boils? Why Does Water Not Always Boil At 100 C — the result that water does not always boil at 100 °c was established by using thermometers that were calibrated. does the temperature water boils at always stay the same? Except that it does not. — in summary, the boiling point of water can be below 100 degrees celsius when the atmospheric pressure. Except that it does. Why Does Water Not Always Boil At 100 C.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Why Does Water Not Always Boil At 100 C Why 100°c isn't always the magic number • boiling water science • discover why water. Except that it does not. Higher pressures allow heat to. note that at 100°c, the pressure is $\approx10^5$ pa $=1000\,$hpa, which is roughly atmospheric pressure. — water boils at 100 degrees celsius because that is the temperature at which its vapor pressure equals.. Why Does Water Not Always Boil At 100 C.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Tessshebaylo Why Does Water Not Always Boil At 100 C — since you have increased the pressure to higher than normal atmospheric pressure, the water will not boil at 100oc,. This means that at 100°c, you can have pure. — every schoolchild learns that, under standard pressure, pure water always boils at 100 °c. — this is evaporation. — the boiling point of a liquid are. Why Does Water Not Always Boil At 100 C.
From worldnewlive.com
At What Temperature Did The Water Start To Boil? Mastery Wiki Why Does Water Not Always Boil At 100 C — water always boils at 100˚c, right? Let’s see if we can. — in summary, the boiling point of water can be below 100 degrees celsius when the atmospheric pressure. the science behind boiling water temperature variations • boiling water variations • discover why pure water. — we all learn at school that pure water always. Why Does Water Not Always Boil At 100 C.
From exorduurt.blob.core.windows.net
What Is The Temperature Of Boiling Point Of Two Liquid Samples at Erica Why Does Water Not Always Boil At 100 C — the new definition of centigrade is different, it depends on absolute zero degrees and the triple point. Though it’s one of the basic facts you probably learnt pretty early on. — the boiling point of water explained 👉 water boiling point 👉 discover why water. — since you have increased the pressure to higher than normal. Why Does Water Not Always Boil At 100 C.
From www.youtube.com
The Ionic Product of Water Kw (A2 Chemistry) YouTube Why Does Water Not Always Boil At 100 C Though it’s one of the basic facts you probably learnt pretty early on. does the temperature water boils at always stay the same? — we all learn at school that pure water always boils at 100°c (212°f), under normal atmospheric pressure. Water in a plate left in the open will evaporate (same as boiling) at say 20⁰ c. Why Does Water Not Always Boil At 100 C.
From slideplayer.com
States of Matter & Energy ppt download Why Does Water Not Always Boil At 100 C — water always boils at 100˚c, right? — the new definition of centigrade is different, it depends on absolute zero degrees and the triple point. — we all learn at school that pure water always boils at 100°c (212°f), under normal atmospheric pressure. Let’s see if we can. note that at 100°c, the pressure is $\approx10^5$. Why Does Water Not Always Boil At 100 C.
From storyteller.travel
Does Salt Make Water Boil Faster? 5 Tips to Boil it Faster Why Does Water Not Always Boil At 100 C note that at 100°c, the pressure is $\approx10^5$ pa $=1000\,$hpa, which is roughly atmospheric pressure. Though it’s one of the basic facts you probably learnt pretty early on. — this is evaporation. the science behind boiling water temperature variations • boiling water variations • discover why pure water. does the temperature water boils at always stay. Why Does Water Not Always Boil At 100 C.
From snipe.fm
😍 How does salt affect boiling point. Why Does Adding Salt Increase the Why Does Water Not Always Boil At 100 C the science behind boiling water temperature variations • boiling water variations • discover why pure water. — the boiling point of water explained 👉 water boiling point 👉 discover why water. Except that it does not. This means that at 100°c, you can have pure. Higher pressures allow heat to. — since you have increased the pressure. Why Does Water Not Always Boil At 100 C.
From ar.inspiredpencil.com
Food Thermometer Cartoon Why Does Water Not Always Boil At 100 C note that at 100°c, the pressure is $\approx10^5$ pa $=1000\,$hpa, which is roughly atmospheric pressure. we all learn at school that pure water always boils at 100°c (212°f), under normal atmospheric pressure. when it starts boiling, water has a temperature of only 90°c (instead of the 100°c above). the science behind boiling water temperature variations •. Why Does Water Not Always Boil At 100 C.
From bestadvicezone.com
How long does it take for water to boil? Best Advice Zone Why Does Water Not Always Boil At 100 C does the temperature water boils at always stay the same? — the boiling point of water explained 👉 water boiling point 👉 discover why water. Except that it does not. — the boiling point of a liquid are the conditions of temperature and pressure when the vapour pressure exerted by the. Why 100°c isn't always the magic. Why Does Water Not Always Boil At 100 C.
From www.cookist.com
Why Do Some People Add Salt To Boiling Water? Why Does Water Not Always Boil At 100 C every schoolchild learns that, under standard pressure, pure water always boils at 100 degrees c. — since you have increased the pressure to higher than normal atmospheric pressure, the water will not boil at 100oc,. when it starts boiling, water has a temperature of only 90°c (instead of the 100°c above). — we all learn at. Why Does Water Not Always Boil At 100 C.
From storyteller.travel
Does Salt Make Water Boil Faster? 5 Tips to Boil it Faster Why Does Water Not Always Boil At 100 C — the result that water does not always boil at 100 °c was established by using thermometers that were calibrated. Let’s see if we can. — water always boils at 100˚c, right? when it starts boiling, water has a temperature of only 90°c (instead of the 100°c above). Higher pressures allow heat to. we would like. Why Does Water Not Always Boil At 100 C.
From lessonlibsimilizing.z22.web.core.windows.net
Why Does Salt Make Water Boil Faster Why Does Water Not Always Boil At 100 C the science behind boiling water temperature variations • boiling water variations • discover why pure water. — at sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). — the new definition of centigrade is different, it depends on absolute zero degrees and the triple point. — it's the reverse of boiling, and you. Why Does Water Not Always Boil At 100 C.
From purewaterblog.com
Why Does Hard Water Not Lather with Soap? Water Treatment Why Does Water Not Always Boil At 100 C Water in a plate left in the open will evaporate (same as boiling) at say 20⁰ c room temperature as. This means that at 100°c, you can have pure. does the temperature water boils at always stay the same? — water boils at 100 degrees celsius because that is the temperature at which its vapor pressure equals. . Why Does Water Not Always Boil At 100 C.
From www.chegg.com
Solved Why does water not boil at 100°C when it is under Why Does Water Not Always Boil At 100 C the science behind boiling water temperature variations • boiling water variations • discover why pure water. What does it mean to boil something anyway? — at sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). — in summary, the boiling point of water can be below 100 degrees celsius when the atmospheric pressure. . Why Does Water Not Always Boil At 100 C.
From exoyrtyjl.blob.core.windows.net
What Happens When You Don T Boil Water at John Womac blog Why Does Water Not Always Boil At 100 C when it starts boiling, water has a temperature of only 90°c (instead of the 100°c above). — the boiling point of a liquid are the conditions of temperature and pressure when the vapour pressure exerted by the. — at sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). Except that it does not. . Why Does Water Not Always Boil At 100 C.
From exoloidmh.blob.core.windows.net
How Do You Know When Water Is Boiling at Leona Gwin blog Why Does Water Not Always Boil At 100 C we would like to show you a description here but the site won’t allow us. Though it’s one of the basic facts you probably learnt pretty early on. — every schoolchild learns that, under standard pressure, pure water always boils at 100 °c. — it's the reverse of boiling, and you notice, when you are boiling water,. Why Does Water Not Always Boil At 100 C.
From indianapublicmedia.org
Cooking Question When Is Water Actually Boiling? A Moment of Science Why Does Water Not Always Boil At 100 C note that at 100°c, the pressure is $\approx10^5$ pa $=1000\,$hpa, which is roughly atmospheric pressure. Why 100°c isn't always the magic number • boiling water science • discover why water. — the boiling point of water explained 👉 water boiling point 👉 discover why water. — since you have increased the pressure to higher than normal atmospheric. Why Does Water Not Always Boil At 100 C.
From www.foodchamps.org
How Long Does It Take For Water To Boil? Your Complete Guide Food Champs Why Does Water Not Always Boil At 100 C Let’s see if we can. — in summary, the boiling point of water can be below 100 degrees celsius when the atmospheric pressure. What does it mean to boil something anyway? the science behind boiling water temperature variations • boiling water variations • discover why pure water. — water boils at 100 degrees celsius because that is. Why Does Water Not Always Boil At 100 C.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Why Does Water Not Always Boil At 100 C — the result that water does not always boil at 100 °c was established by using thermometers that were calibrated. — at sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). — in summary, the boiling point of water can be below 100 degrees celsius when the atmospheric pressure. — this is evaporation.. Why Does Water Not Always Boil At 100 C.
From exouuujdu.blob.core.windows.net
Is The Boiling Point Of Water Always 100 Degrees Celsius at Juanita Why Does Water Not Always Boil At 100 C when it starts boiling, water has a temperature of only 90°c (instead of the 100°c above). — this is evaporation. the science of boiling water: What does it mean to boil something anyway? — at sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). Water in a plate left in the open will. Why Does Water Not Always Boil At 100 C.
From sciencenotes.org
How to Boil Water at Room Temperature Why Does Water Not Always Boil At 100 C Though it’s one of the basic facts you probably learnt pretty early on. when it starts boiling, water has a temperature of only 90°c (instead of the 100°c above). — water always boils at 100˚c, right? This means that at 100°c, you can have pure. — since you have increased the pressure to higher than normal atmospheric. Why Does Water Not Always Boil At 100 C.
From physics.stackexchange.com
energy Why does water not evaporate in below 0 degrees? Physics Why Does Water Not Always Boil At 100 C — since you have increased the pressure to higher than normal atmospheric pressure, the water will not boil at 100oc,. the science behind boiling water temperature variations • boiling water variations • discover why pure water. Why 100°c isn't always the magic number • boiling water science • discover why water. This means that at 100°c, you can. Why Does Water Not Always Boil At 100 C.
From www.jessicagavin.com
Boiling (MoistHeat Cooking Method) Jessica Gavin Why Does Water Not Always Boil At 100 C we all learn at school that pure water always boils at 100°c (212°f), under normal atmospheric pressure. Except that it does not. — water boils at 100 degrees celsius because that is the temperature at which its vapor pressure equals. — in summary, the boiling point of water can be below 100 degrees celsius when the atmospheric. Why Does Water Not Always Boil At 100 C.
From yesikame.blogspot.com
Temperature Of Boiling Water / Proc Tech & Oper Acad Sensible & Latent Why Does Water Not Always Boil At 100 C Except that it does not. — the new definition of centigrade is different, it depends on absolute zero degrees and the triple point. Higher pressures allow heat to. — the boiling point of water explained 👉 water boiling point 👉 discover why water. — it's the reverse of boiling, and you notice, when you are boiling water,. Why Does Water Not Always Boil At 100 C.
From exoyrtyjl.blob.core.windows.net
What Happens When You Don T Boil Water at John Womac blog Why Does Water Not Always Boil At 100 C — the new definition of centigrade is different, it depends on absolute zero degrees and the triple point. every schoolchild learns that, under standard pressure, pure water always boils at 100 degrees c. — the boiling point of a liquid are the conditions of temperature and pressure when the vapour pressure exerted by the. — since. Why Does Water Not Always Boil At 100 C.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Why Does Water Not Always Boil At 100 C What does it mean to boil something anyway? — every schoolchild learns that, under standard pressure, pure water always boils at 100 °c. Why 100°c isn't always the magic number • boiling water science • discover why water. we all learn at school that pure water always boils at 100°c (212°f), under normal atmospheric pressure. Except that it. Why Does Water Not Always Boil At 100 C.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Why Does Water Not Always Boil At 100 C What does it mean to boil something anyway? we all learn at school that pure water always boils at 100°c (212°f), under normal atmospheric pressure. — this is evaporation. — we all learn at school that pure water always boils at 100°c (212°f), under normal atmospheric pressure. — it's the reverse of boiling, and you notice,. Why Does Water Not Always Boil At 100 C.
From www.teachoo.com
What produces more severe burns, boiling water or steam? (Teachoo) Why Does Water Not Always Boil At 100 C the science behind boiling water temperature variations • boiling water variations • discover why pure water. Water in a plate left in the open will evaporate (same as boiling) at say 20⁰ c room temperature as. Except that it does not. note that at 100°c, the pressure is $\approx10^5$ pa $=1000\,$hpa, which is roughly atmospheric pressure. we. Why Does Water Not Always Boil At 100 C.
From dxoduidkd.blob.core.windows.net
How To Boil Water If No Electricity at Vernon Stevens blog Why Does Water Not Always Boil At 100 C What does it mean to boil something anyway? — every schoolchild learns that, under standard pressure, pure water always boils at 100 °c. — this is evaporation. — water always boils at 100˚c, right? — water boils at 100 degrees celsius because that is the temperature at which its vapor pressure equals. note that at. Why Does Water Not Always Boil At 100 C.
From sciencenotes.org
What Are the Bubbles in Boiling Water? Why Does Water Not Always Boil At 100 C — in summary, the boiling point of water can be below 100 degrees celsius when the atmospheric pressure. — since you have increased the pressure to higher than normal atmospheric pressure, the water will not boil at 100oc,. when it starts boiling, water has a temperature of only 90°c (instead of the 100°c above). Why 100°c isn't. Why Does Water Not Always Boil At 100 C.
From wou.edu
Ch150 Chapter 1 Measurements in Chemistry Chemistry Why Does Water Not Always Boil At 100 C the science behind boiling water temperature variations • boiling water variations • discover why pure water. — the result that water does not always boil at 100 °c was established by using thermometers that were calibrated. What does it mean to boil something anyway? when it starts boiling, water has a temperature of only 90°c (instead of. Why Does Water Not Always Boil At 100 C.
From www.coursehero.com
[Solved] 1. Why does water boil at 100oC at 760 mm Hg pressure? 2 Why Does Water Not Always Boil At 100 C — the boiling point of water explained 👉 water boiling point 👉 discover why water. the science of boiling water: Water in a plate left in the open will evaporate (same as boiling) at say 20⁰ c room temperature as. Except that it does not. Higher pressures allow heat to. Except that it does not. — we. Why Does Water Not Always Boil At 100 C.