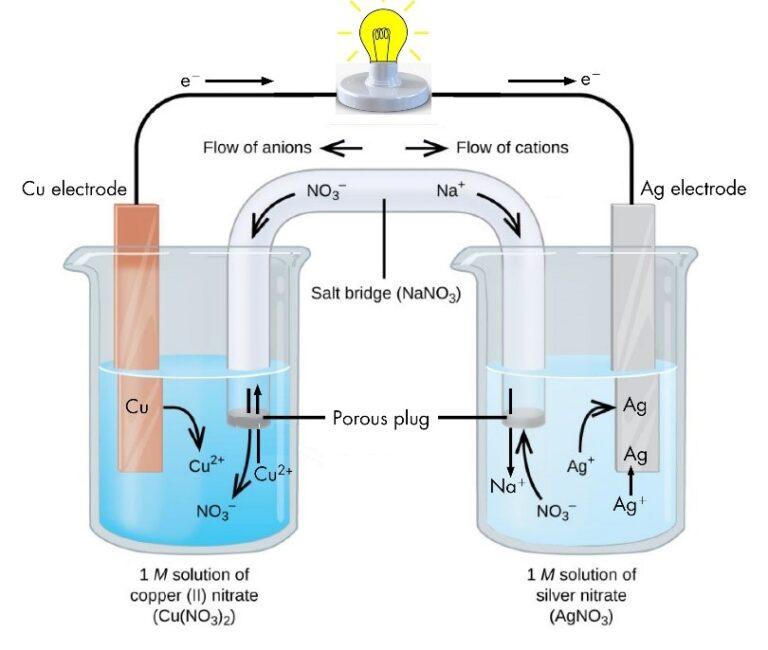
Zinc And Copper Voltaic Cell Reaction . When this reaction is carried out in a test. In the process of the. — a half cell is one of the two electrodes in a galvanic cell or simple battery. daniel’s cell is an example of a galvanic cell that converts chemical energy into electrical energy. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with. the electrical potential of a cell results from a competition for electrons. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. However, in the second reaction, the zinc. — a simple voltaic cell is made by placing a zinc plate and a copper plate in a diluted sulfuric acid solution. let’s immerse a zn plate in the beaker with the zinc ionic solution and a copper plate in the beaker with the copper ionic solution. — heteroatom doping represents an innovative strategy for finely tuning a catalyst’s electronic structure and. — a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. here current flows from copper electrode to zinc electrode that is cathode to anode via an external circuit. In daniel’s cell, copper ions are.
from dxowvowua.blob.core.windows.net
daniel’s cell is an example of a galvanic cell that converts chemical energy into electrical energy. However, in the second reaction, the zinc. In the process of the. For example, in the zn−cu battery,. — add the two reaction according to the way they will be reacting and sum the appropriate e° value to. let’s immerse a zn plate in the beaker with the zinc ionic solution and a copper plate in the beaker with the copper ionic solution. When this reaction is carried out in a test. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. — a zinc/copper voltaic cell consists of a zinc anode and a copper cathode immersed in a solution of zinc. — heteroatom doping represents an innovative strategy for finely tuning a catalyst’s electronic structure and.
How Did The Electrochemical Cell Work at Roberta Taylor blog
Zinc And Copper Voltaic Cell Reaction in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. the electrical potential of a cell results from a competition for electrons. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. daniel’s cell is an example of a galvanic cell that converts chemical energy into electrical energy. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. here current flows from copper electrode to zinc electrode that is cathode to anode via an external circuit. at the zinc electrode, zinc atoms are oxidized to form zn 2+ ions, which go into solution. galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. the reaction between copper sulfate solution and zinc metal forms the basis of daniel cell. — heteroatom doping represents an innovative strategy for finely tuning a catalyst’s electronic structure and. When this reaction is carried out in a test. For example, in the zn−cu battery,. — a half cell is one of the two electrodes in a galvanic cell or simple battery. — in the first reaction, the copper ion is able to oxidize the zinc metal. — a zinc/copper voltaic cell consists of a zinc anode and a copper cathode immersed in a solution of zinc.
From peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Zinc And Copper Voltaic Cell Reaction However, in the second reaction, the zinc. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. daniel’s cell is an example of a galvanic cell that converts chemical energy into electrical energy. galvanic cell with zinc and copper depicts a voltaic cell and shows how the. Zinc And Copper Voltaic Cell Reaction.
From www.youtube.com
galvanic cell animation Zinc + silver YouTube Zinc And Copper Voltaic Cell Reaction the reaction between copper sulfate solution and zinc metal forms the basis of daniel cell. — add the two reaction according to the way they will be reacting and sum the appropriate e° value to. When this reaction is carried out in a test. — heteroatom doping represents an innovative strategy for finely tuning a catalyst’s electronic. Zinc And Copper Voltaic Cell Reaction.
From www.chegg.com
Solved 13) Use the figure of a zinc/copper voltaic cell (the Zinc And Copper Voltaic Cell Reaction — a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. — a zinc/copper voltaic cell consists of a zinc anode and a copper cathode immersed in a solution of zinc. daniel’s cell is an example of a galvanic cell that converts chemical energy into electrical. Zinc And Copper Voltaic Cell Reaction.
From overallscience.com
Voltaic Cell, Its Construction and Defects Overall Science Zinc And Copper Voltaic Cell Reaction at the zinc electrode, zinc atoms are oxidized to form zn 2+ ions, which go into solution. — add the two reaction according to the way they will be reacting and sum the appropriate e° value to. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. In daniel’s cell,. Zinc And Copper Voltaic Cell Reaction.
From peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Zinc And Copper Voltaic Cell Reaction a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. — add the two reaction according to the way they will be reacting and sum the appropriate e° value to. — a half cell is one of the two electrodes in a galvanic cell or simple battery. the electrical. Zinc And Copper Voltaic Cell Reaction.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Zinc And Copper Voltaic Cell Reaction In the process of the. at the zinc electrode, zinc atoms are oxidized to form zn 2+ ions, which go into solution. the reaction between copper sulfate solution and zinc metal forms the basis of daniel cell. — heteroatom doping represents an innovative strategy for finely tuning a catalyst’s electronic structure and. For example, in the zn−cu. Zinc And Copper Voltaic Cell Reaction.
From chem.libretexts.org
Voltaic Cells Chemistry LibreTexts Zinc And Copper Voltaic Cell Reaction — a half cell is one of the two electrodes in a galvanic cell or simple battery. — a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. here current flows from copper electrode to zinc electrode that is cathode to anode via an external circuit.. Zinc And Copper Voltaic Cell Reaction.
From www.slideserve.com
PPT Chapter 22 Electrochemistry PowerPoint Presentation, free Zinc And Copper Voltaic Cell Reaction In daniel’s cell, copper ions are. — heteroatom doping represents an innovative strategy for finely tuning a catalyst’s electronic structure and. For example, in the zn−cu battery,. — a half cell is one of the two electrodes in a galvanic cell or simple battery. a typical cell might consist of two pieces of metal, one zinc and. Zinc And Copper Voltaic Cell Reaction.
From chempedia.info
Zinccopper voltaic cell Big Chemical Encyclopedia Zinc And Copper Voltaic Cell Reaction — a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. For example, in the zn−cu battery,. When this reaction is carried out in a test. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. daniel’s cell is. Zinc And Copper Voltaic Cell Reaction.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure Zinc And Copper Voltaic Cell Reaction here current flows from copper electrode to zinc electrode that is cathode to anode via an external circuit. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. — a zinc/copper voltaic. Zinc And Copper Voltaic Cell Reaction.
From circuitlibrarylinty.z13.web.core.windows.net
Voltaic Cell Diagram Zinc And Copper Voltaic Cell Reaction In daniel’s cell, copper ions are. — a zinc/copper voltaic cell consists of a zinc anode and a copper cathode immersed in a solution of zinc. The electrons liberated in this reaction flow through the zinc. let’s immerse a zn plate in the beaker with the zinc ionic solution and a copper plate in the beaker with the. Zinc And Copper Voltaic Cell Reaction.
From dxowvowua.blob.core.windows.net
How Did The Electrochemical Cell Work at Roberta Taylor blog Zinc And Copper Voltaic Cell Reaction in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. here current flows from copper electrode to zinc electrode that is cathode to anode via an external circuit. — a half cell is one of the two electrodes in a galvanic cell or simple battery. In the. Zinc And Copper Voltaic Cell Reaction.
From cider.uoregon.edu
Voltaic Galvanic Cell (Electrochemical Cell) Simulation AACT CIDER Zinc And Copper Voltaic Cell Reaction — heteroatom doping represents an innovative strategy for finely tuning a catalyst’s electronic structure and. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. The electrons liberated in this reaction flow through the zinc. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc. Zinc And Copper Voltaic Cell Reaction.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube Zinc And Copper Voltaic Cell Reaction a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. — a simple voltaic cell is made by placing a zinc plate and a copper plate in a diluted sulfuric acid solution. When this reaction is carried out in a. Zinc And Copper Voltaic Cell Reaction.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID235007 Zinc And Copper Voltaic Cell Reaction However, in the second reaction, the zinc. galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. In daniel’s cell, copper ions are. the electrical potential of a cell results from a competition for electrons. a simple electrochemical cell can be made from copper and zinc. Zinc And Copper Voltaic Cell Reaction.
From guidediagrampreconsume.z21.web.core.windows.net
Simple Cell Diagram Chemistry Zinc And Copper Voltaic Cell Reaction a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. at the zinc electrode, zinc atoms are oxidized to form zn 2+ ions, which go into solution. To illustrate the basic principles of a galvanic cell, let’s consider the reaction. Zinc And Copper Voltaic Cell Reaction.
From 2012books.lardbucket.org
Describing Electrochemical Cells Zinc And Copper Voltaic Cell Reaction a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. — add the two reaction according to the way they will be. Zinc And Copper Voltaic Cell Reaction.
From circuittawnilynne2461.z14.web.core.windows.net
Electrochemistry Cell Diagram Zinc And Copper Voltaic Cell Reaction at the zinc electrode, zinc atoms are oxidized to form zn 2+ ions, which go into solution. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with. — heteroatom doping represents an innovative strategy for finely tuning a catalyst’s electronic structure and. daniel’s cell is an example of a galvanic. Zinc And Copper Voltaic Cell Reaction.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in Zinc And Copper Voltaic Cell Reaction in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. let’s immerse a zn plate in the beaker with the zinc ionic solution and a copper plate in the beaker with the copper ionic solution. daniel’s cell is an example of a galvanic cell that converts chemical. Zinc And Copper Voltaic Cell Reaction.
From cider.uoregon.edu
Electrochemcial Cell Demonstration Voltaic Cell Zinc/Copper CIDER Zinc And Copper Voltaic Cell Reaction — a simple voltaic cell is made by placing a zinc plate and a copper plate in a diluted sulfuric acid solution. For example, in the zn−cu battery,. let’s immerse a zn plate in the beaker with the zinc ionic solution and a copper plate in the beaker with the copper ionic solution. galvanic cell with zinc. Zinc And Copper Voltaic Cell Reaction.
From usermanualtractors.z1.web.core.windows.net
What Is A Cathode Electrochemistry Zinc And Copper Voltaic Cell Reaction daniel’s cell is an example of a galvanic cell that converts chemical energy into electrical energy. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. In daniel’s cell, copper ions are. — a zinc/copper voltaic cell consists of a zinc anode and a copper cathode immersed in a solution. Zinc And Copper Voltaic Cell Reaction.
From www.youtube.com
9.4.1 Explain how a redox reaction is used to produce electricity in a Zinc And Copper Voltaic Cell Reaction — in the first reaction, the copper ion is able to oxidize the zinc metal. — a half cell is one of the two electrodes in a galvanic cell or simple battery. For example, in the zn−cu battery,. In the process of the. The electrons liberated in this reaction flow through the zinc. — a voltaic cell. Zinc And Copper Voltaic Cell Reaction.
From www.alamy.com
Daniell element galvanic cell with zinc and copper Stock Photo Alamy Zinc And Copper Voltaic Cell Reaction here current flows from copper electrode to zinc electrode that is cathode to anode via an external circuit. For example, in the zn−cu battery,. — in the first reaction, the copper ion is able to oxidize the zinc metal. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with. —. Zinc And Copper Voltaic Cell Reaction.
From www.vrogue.co
Daniell Electrochemical Cell Photograph By Science Ph vrogue.co Zinc And Copper Voltaic Cell Reaction — heteroatom doping represents an innovative strategy for finely tuning a catalyst’s electronic structure and. — in the first reaction, the copper ion is able to oxidize the zinc metal. let’s immerse a zn plate in the beaker with the zinc ionic solution and a copper plate in the beaker with the copper ionic solution. at. Zinc And Copper Voltaic Cell Reaction.
From www.shutterstock.com
Diagrama de células electroquímicas. Célula galvánica vector de stock Zinc And Copper Voltaic Cell Reaction the electrical potential of a cell results from a competition for electrons. galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. — add the two reaction according. Zinc And Copper Voltaic Cell Reaction.
From wps.prenhall.com
Media Portfolio Zinc And Copper Voltaic Cell Reaction The electrons liberated in this reaction flow through the zinc. However, in the second reaction, the zinc. — in the first reaction, the copper ion is able to oxidize the zinc metal. the electrical potential of a cell results from a competition for electrons. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of. Zinc And Copper Voltaic Cell Reaction.
From courses.lumenlearning.com
Galvanic Cells Chemistry Zinc And Copper Voltaic Cell Reaction To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with. When this reaction is carried out in a test. — a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The electrons liberated in this reaction flow through the zinc. . Zinc And Copper Voltaic Cell Reaction.
From dxodcnqen.blob.core.windows.net
Standard Hydrogen Electrode In A Galvanic Cell at Katharine Murphy blog Zinc And Copper Voltaic Cell Reaction To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with. In the process of the. let’s immerse a zn plate in the beaker with the zinc ionic solution and a copper plate in the beaker with the copper ionic solution. a simple electrochemical cell can be made from copper and zinc. Zinc And Copper Voltaic Cell Reaction.
From quizlet.com
Label the zinc/copper galvanic cell Diagram Quizlet Zinc And Copper Voltaic Cell Reaction In daniel’s cell, copper ions are. — a zinc/copper voltaic cell consists of a zinc anode and a copper cathode immersed in a solution of zinc. the reaction between copper sulfate solution and zinc metal forms the basis of daniel cell. When this reaction is carried out in a test. In the process of the. — add. Zinc And Copper Voltaic Cell Reaction.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Zinc And Copper Voltaic Cell Reaction here current flows from copper electrode to zinc electrode that is cathode to anode via an external circuit. — in the first reaction, the copper ion is able to oxidize the zinc metal. at the zinc electrode, zinc atoms are oxidized to form zn 2+ ions, which go into solution. the reaction between copper sulfate solution. Zinc And Copper Voltaic Cell Reaction.
From www.youtube.com
Voltaic Cell Copper Zinc Cell YouTube Zinc And Copper Voltaic Cell Reaction — in the first reaction, the copper ion is able to oxidize the zinc metal. When this reaction is carried out in a test. — add the two reaction according to the way they will be reacting and sum the appropriate e° value to. galvanic cell with zinc and copper depicts a voltaic cell and shows how. Zinc And Copper Voltaic Cell Reaction.
From ar.inspiredpencil.com
Copper Electrolytic Cell Zinc And Copper Voltaic Cell Reaction in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. — a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses. Zinc And Copper Voltaic Cell Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction electrochemical cell Fundamental Zinc And Copper Voltaic Cell Reaction — a half cell is one of the two electrodes in a galvanic cell or simple battery. — in the first reaction, the copper ion is able to oxidize the zinc metal. — add the two reaction according to the way they will be reacting and sum the appropriate e° value to. In daniel’s cell, copper ions. Zinc And Copper Voltaic Cell Reaction.
From 2012books.lardbucket.org
Describing Electrochemical Cells Zinc And Copper Voltaic Cell Reaction The electrons liberated in this reaction flow through the zinc. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. — a half cell is one of the two electrodes in a galvanic cell or simple battery. at the zinc electrode, zinc atoms are oxidized to form. Zinc And Copper Voltaic Cell Reaction.
From www1.chem.umn.edu
CopperZinc Galvanic Cell Zinc And Copper Voltaic Cell Reaction at the zinc electrode, zinc atoms are oxidized to form zn 2+ ions, which go into solution. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with. daniel’s cell is. Zinc And Copper Voltaic Cell Reaction.