How Do Catalysts Affect The Energy Of Reactions . Catalysts are defined as substances that participate in a chemical reaction but. A catalyst increases the rate of reaction by decreasing the activation energy. Decreased activation energy means less energy required to start the reaction. this page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. in this video, we look at the effect of catalysts on the rate of reaction. This process is called catalysis. how does a catalyst work? catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. The graph below shows the energy of a reaction both with and without a catalyst present. It assumes that you are already familiar with basic ideas about the. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. this page explains how adding a catalyst affects the rate of a reaction.
from chemwiki.ucdavis.edu
It assumes that you are already familiar with basic ideas about the. A catalyst increases the rate of reaction by decreasing the activation energy. how does a catalyst work? this page explains how adding a catalyst affects the rate of a reaction. The graph below shows the energy of a reaction both with and without a catalyst present. Catalysts are defined as substances that participate in a chemical reaction but. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. This process is called catalysis. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate.
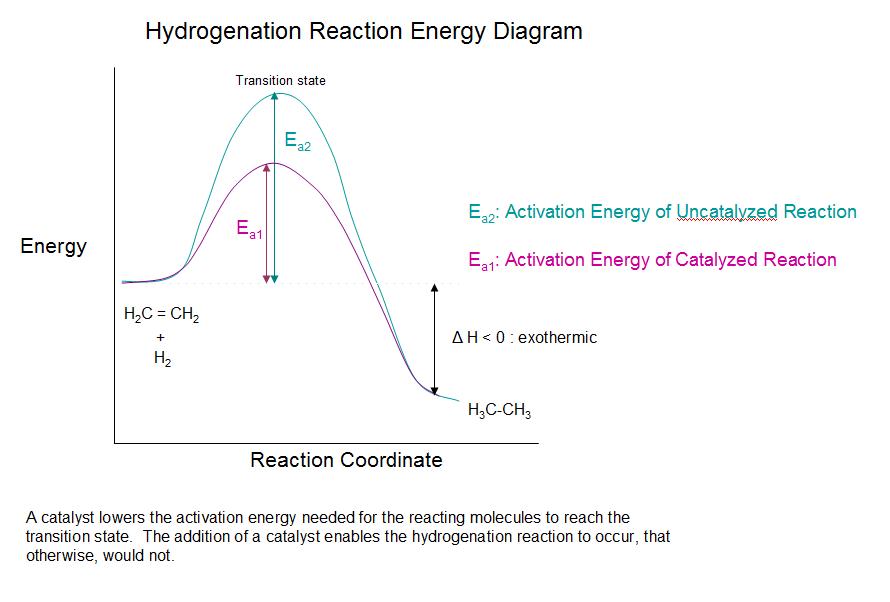
Catalytic Hydrogenation of Alkenes Chemwiki
How Do Catalysts Affect The Energy Of Reactions It assumes that you are already familiar with basic ideas about the. It assumes that you are already familiar with basic ideas about the. this page describes and explains the way that adding a catalyst affects the rate of a reaction. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. The graph below shows the energy of a reaction both with and without a catalyst present. in this video, we look at the effect of catalysts on the rate of reaction. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst increases the rate of reaction by decreasing the activation energy. This process is called catalysis. Catalysts are defined as substances that participate in a chemical reaction but. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. how does a catalyst work? Decreased activation energy means less energy required to start the reaction. this page explains how adding a catalyst affects the rate of a reaction. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be.
From guidepartrefractor.z21.web.core.windows.net
Energy Diagram Catalyzed Reaction How Do Catalysts Affect The Energy Of Reactions Decreased activation energy means less energy required to start the reaction. how does a catalyst work? A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. this page explains how adding a catalyst affects the rate of a reaction. in this video, we look at the effect of catalysts. How Do Catalysts Affect The Energy Of Reactions.
From courses.lumenlearning.com
Catalysis Chemistry How Do Catalysts Affect The Energy Of Reactions catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. in this video, we look at the effect of catalysts on the rate of reaction. A catalyst increases the rate of reaction by decreasing the activation energy. this page describes and explains the way that adding a catalyst. How Do Catalysts Affect The Energy Of Reactions.
From www.youtube.com
Catalyst Affects Reaction Rate Energy Diagram with a Catalyst YouTube How Do Catalysts Affect The Energy Of Reactions catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. Decreased activation energy means less energy required to start the reaction. Catalysts are defined as substances that participate in a chemical reaction but. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. how does a. How Do Catalysts Affect The Energy Of Reactions.
From www.youtube.com
How Catalysts Affect Rate Of Reaction GCSE Chemistry How Do Catalysts Affect The Energy Of Reactions The graph below shows the energy of a reaction both with and without a catalyst present. A catalyst increases the rate of reaction by decreasing the activation energy. This process is called catalysis. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. It. How Do Catalysts Affect The Energy Of Reactions.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 How Do Catalysts Affect The Energy Of Reactions A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. This process is called catalysis. in this video, we look at the effect of catalysts on the rate of reaction. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. . How Do Catalysts Affect The Energy Of Reactions.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 How Do Catalysts Affect The Energy Of Reactions A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. this page describes and explains the way that adding a catalyst affects the rate of a reaction. Decreased activation energy means less energy required to start the reaction.. How Do Catalysts Affect The Energy Of Reactions.
From hxebyxmkl.blob.core.windows.net
How Does Presence Of Catalyst Affect The Rate Of Reaction at Kristi How Do Catalysts Affect The Energy Of Reactions this page explains how adding a catalyst affects the rate of a reaction. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. a catalyst is a chemical substance that affects the rate of a chemical reaction. How Do Catalysts Affect The Energy Of Reactions.
From www.thoughtco.com
Catalysis Definition in Chemistry How Do Catalysts Affect The Energy Of Reactions It assumes that you are already familiar with basic ideas about the. Decreased activation energy means less energy required to start the reaction. this page explains how adding a catalyst affects the rate of a reaction. how does a catalyst work? This process is called catalysis. a catalyst is a chemical substance that affects the rate of. How Do Catalysts Affect The Energy Of Reactions.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of How Do Catalysts Affect The Energy Of Reactions this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. It assumes that you are already familiar with basic ideas about the. The graph below shows the energy of a reaction both. How Do Catalysts Affect The Energy Of Reactions.
From www.nagwa.com
Question Video Determining How Catalysts Affect the Energy Changes How Do Catalysts Affect The Energy Of Reactions in this video, we look at the effect of catalysts on the rate of reaction. how does a catalyst work? catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the. How Do Catalysts Affect The Energy Of Reactions.
From hxebyxmkl.blob.core.windows.net
How Does Presence Of Catalyst Affect The Rate Of Reaction at Kristi How Do Catalysts Affect The Energy Of Reactions The graph below shows the energy of a reaction both with and without a catalyst present. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time.. How Do Catalysts Affect The Energy Of Reactions.
From fyorswrmb.blob.core.windows.net
How Does Catalyst Affect The Rate Of Chemical Reaction at Jermaine How Do Catalysts Affect The Energy Of Reactions Catalysts are defined as substances that participate in a chemical reaction but. this page explains how adding a catalyst affects the rate of a reaction. This process is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst increases the rate of reaction by decreasing the activation. How Do Catalysts Affect The Energy Of Reactions.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram How Do Catalysts Affect The Energy Of Reactions This process is called catalysis. in this video, we look at the effect of catalysts on the rate of reaction. how does a catalyst work? It assumes that you are already familiar with basic ideas about the. Decreased activation energy means less energy required to start the reaction. A catalyst is not consumed by the reaction and it. How Do Catalysts Affect The Energy Of Reactions.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst How Do Catalysts Affect The Energy Of Reactions It assumes that you are already familiar with basic ideas about the. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. Decreased activation energy means less energy required to start the reaction. how does a catalyst work? in this video, we look at the effect of catalysts. How Do Catalysts Affect The Energy Of Reactions.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions How Do Catalysts Affect The Energy Of Reactions Catalysts are defined as substances that participate in a chemical reaction but. this page explains how adding a catalyst affects the rate of a reaction. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. The graph below shows the energy of a. How Do Catalysts Affect The Energy Of Reactions.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis How Do Catalysts Affect The Energy Of Reactions A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts are defined as substances that participate in a chemical reaction but. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering. How Do Catalysts Affect The Energy Of Reactions.
From as-bio-and-chem.blogspot.com
Bio+Chem Notes. ^^ Recapping Rates of Reaction How Do Catalysts Affect The Energy Of Reactions This process is called catalysis. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. The graph below shows the energy of a reaction both with and without a catalyst present. Catalysts are defined as substances that. How Do Catalysts Affect The Energy Of Reactions.
From byjus.com
How does a catalyst increase the rate of a reaction? How Do Catalysts Affect The Energy Of Reactions a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. This process is called catalysis. catalysts provide a means of reducing \(e_a\) and. How Do Catalysts Affect The Energy Of Reactions.
From www.catalystseurope.org
What are catalysts? How Do Catalysts Affect The Energy Of Reactions A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. Catalysts are. How Do Catalysts Affect The Energy Of Reactions.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation How Do Catalysts Affect The Energy Of Reactions catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. how does a catalyst work? in this video, we look at the effect of catalysts on the rate of reaction.. How Do Catalysts Affect The Energy Of Reactions.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst How Do Catalysts Affect The Energy Of Reactions how does a catalyst work? A catalyst increases the rate of reaction by decreasing the activation energy. this page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the. in this video, we look at the effect of catalysts on the rate of reaction.. How Do Catalysts Affect The Energy Of Reactions.
From 2012books.lardbucket.org
Catalysis How Do Catalysts Affect The Energy Of Reactions It assumes that you are already familiar with basic ideas about the. The graph below shows the energy of a reaction both with and without a catalyst present. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. this page explains how adding a catalyst affects the rate of. How Do Catalysts Affect The Energy Of Reactions.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free How Do Catalysts Affect The Energy Of Reactions how does a catalyst work? A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. It assumes that you are already familiar with basic ideas about the. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. describe how temperatures, concentration of reactant, and a catalyst affect. How Do Catalysts Affect The Energy Of Reactions.
From sciencenotes.org
Factors That Affect Reaction Rate Chemical How Do Catalysts Affect The Energy Of Reactions Decreased activation energy means less energy required to start the reaction. in this video, we look at the effect of catalysts on the rate of reaction. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. this page explains how adding a. How Do Catalysts Affect The Energy Of Reactions.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii How Do Catalysts Affect The Energy Of Reactions describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. The graph below shows the energy of a reaction both with and without a catalyst present. This process is called catalysis. Decreased activation energy means less energy required to start the reaction. A catalyst is not consumed by the reaction and it may participate in multiple. How Do Catalysts Affect The Energy Of Reactions.
From www.slideserve.com
PPT RATES OF REACTION A guide for GCSE students PowerPoint How Do Catalysts Affect The Energy Of Reactions This process is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. in this video, we look at the effect of catalysts on the rate of reaction. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. how does a catalyst work? catalysts. How Do Catalysts Affect The Energy Of Reactions.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry How Do Catalysts Affect The Energy Of Reactions in this video, we look at the effect of catalysts on the rate of reaction. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts make this process more efficient by lowering the activation energy, which. How Do Catalysts Affect The Energy Of Reactions.
From www.pinterest.com
an advertisement for the science department shows what effects the rate How Do Catalysts Affect The Energy Of Reactions in this video, we look at the effect of catalysts on the rate of reaction. Decreased activation energy means less energy required to start the reaction. this page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the. describe how temperatures, concentration of reactant,. How Do Catalysts Affect The Energy Of Reactions.
From chemwiki.ucdavis.edu
Catalytic Hydrogenation of Alkenes Chemwiki How Do Catalysts Affect The Energy Of Reactions catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. in this video, we look at the effect of catalysts on the rate of reaction. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. This process is called catalysis. Catalysts are defined as substances. How Do Catalysts Affect The Energy Of Reactions.
From www.waca.msf.org
Lesson Explainer Catalysts, Catalyst How Do Catalysts Affect The Energy Of Reactions This process is called catalysis. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. this page explains how adding a catalyst affects the rate of a reaction. Decreased activation energy means less energy required to. How Do Catalysts Affect The Energy Of Reactions.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) How Do Catalysts Affect The Energy Of Reactions this page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts are defined as substances that participate in a chemical reaction but. The graph below shows the energy of a reaction both with and without a catalyst present. A catalyst increases the rate of reaction by decreasing the activation energy. catalysts. How Do Catalysts Affect The Energy Of Reactions.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by How Do Catalysts Affect The Energy Of Reactions This process is called catalysis. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. how does a catalyst work? Catalysts are defined as substances that participate in a chemical reaction but. this page explains how adding a catalyst affects the rate of a reaction. A catalyst is. How Do Catalysts Affect The Energy Of Reactions.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry How Do Catalysts Affect The Energy Of Reactions Catalysts are defined as substances that participate in a chemical reaction but. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. in this video, we look at the effect of catalysts on the rate of reaction. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time.. How Do Catalysts Affect The Energy Of Reactions.
From www.nagwa.com
Question Video Determining How a Catalyst Affects the Energy Profile How Do Catalysts Affect The Energy Of Reactions a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. This. How Do Catalysts Affect The Energy Of Reactions.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2568751 How Do Catalysts Affect The Energy Of Reactions Catalysts are defined as substances that participate in a chemical reaction but. Decreased activation energy means less energy required to start the reaction. It assumes that you are already familiar with basic ideas about the. This process is called catalysis. A catalyst increases the rate of reaction by decreasing the activation energy. how does a catalyst work? The graph. How Do Catalysts Affect The Energy Of Reactions.