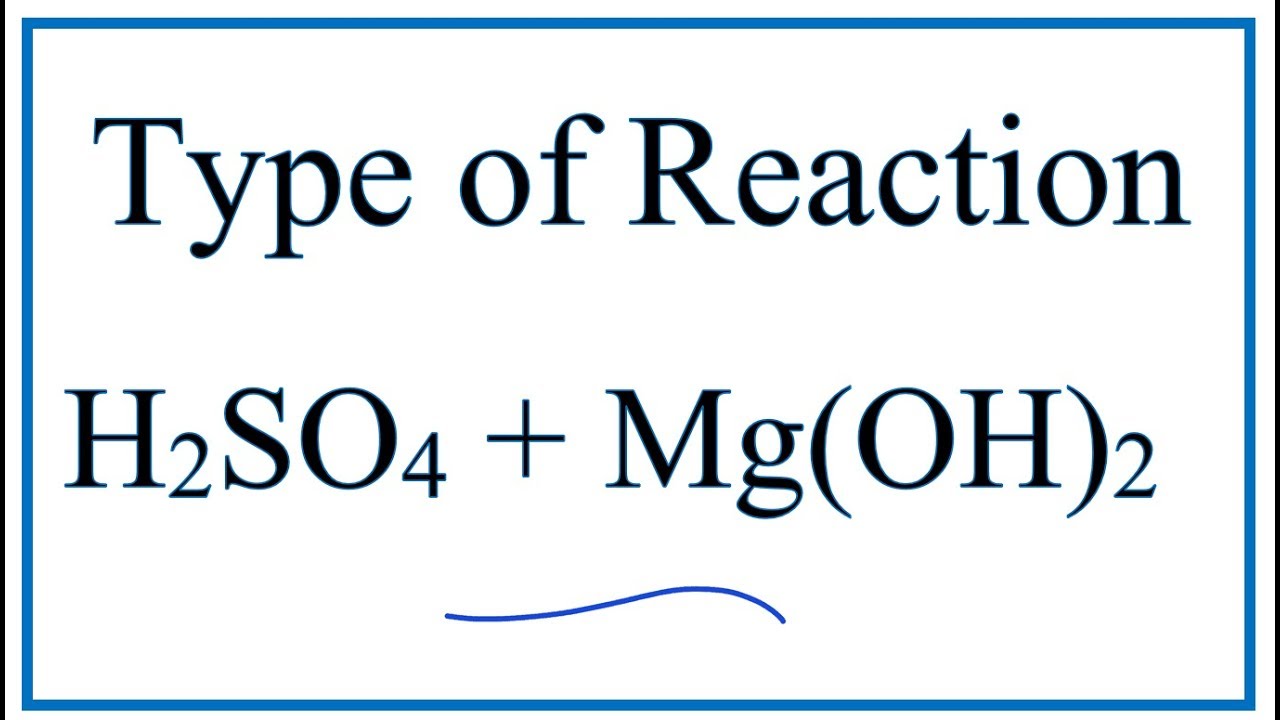
Sulphuric Acid And Magnesium Hydroxide Formula . If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between. if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. enter an equation of an ionic chemical equation and press the balance button. magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. The solutions involved are colorless. The balanced equation will be calculated along.
from w20.b2m.cz
The balanced equation will be calculated along. The solutions involved are colorless. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. enter an equation of an ionic chemical equation and press the balance button. If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between.
Mg Oh 2 H2so4 EDUCA
Sulphuric Acid And Magnesium Hydroxide Formula how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: The balanced equation will be calculated along. The solutions involved are colorless. enter an equation of an ionic chemical equation and press the balance button. magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between.
From www.numerade.com
SOLVEDIf 0.605 g of magnesium hydroxide reacts with 1.00 g of sulfuric Sulphuric Acid And Magnesium Hydroxide Formula The balanced equation will be calculated along. magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. enter an equation of an ionic chemical equation and press the balance button. how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. to determine the formula of the salt formed, write the balanced. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.youtube.com
How to Balance H2SO4 + Mg(OH)2 = H2O + MgSO4 (Sulfuric acid + Magnesium Sulphuric Acid And Magnesium Hydroxide Formula magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: the chemical equation shows magnesium (mg). Sulphuric Acid And Magnesium Hydroxide Formula.
From royce-has-larson.blogspot.com
Magnesium Sulfuric Acid RoycehasLarson Sulphuric Acid And Magnesium Hydroxide Formula The solutions involved are colorless. If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. The balanced equation will be calculated along. how to balance h2so4 + mg. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.slideshare.net
Chemical Reactions Sulphuric Acid And Magnesium Hydroxide Formula The solutions involved are colorless. enter an equation of an ionic chemical equation and press the balance button. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. if the base is a metal hydroxide, then the general formula for the reaction of. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.numerade.com
SOLVED which salt is formed when sulphuric acid and magnesium Sulphuric Acid And Magnesium Hydroxide Formula magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. The solutions involved are colorless. enter an equation of an ionic chemical equation and press the balance button. to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between. how to balance h2so4 + mg (oh)2 = h2o. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.gauthmath.com
Solved The equation shows the reaction between magnesium and sulfuric Sulphuric Acid And Magnesium Hydroxide Formula if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. enter an equation of an ionic chemical equation and. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.toppr.com
How the following substance will dissociate to produce ions in their Sulphuric Acid And Magnesium Hydroxide Formula to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between. enter an equation of an ionic chemical equation and press the balance button. how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. If enough magnesium is used, magnesium sulfate drops out of solution to. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.slideserve.com
PPT DO NOW!! PowerPoint Presentation, free download ID1560113 Sulphuric Acid And Magnesium Hydroxide Formula how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: the chemical equation shows magnesium (mg) and. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.numerade.com
SOLVEDIf 1.00 g of magnesium hydroxide reacts with 0.605 g of sulfuric Sulphuric Acid And Magnesium Hydroxide Formula If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: enter an equation of an ionic chemical equation and press the balance button. how to balance h2so4. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.numerade.com
SOLVED Magnesium reacts with sulfuric acid according to the following Sulphuric Acid And Magnesium Hydroxide Formula The solutions involved are colorless. to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between. enter an equation of an ionic chemical equation and press the balance button. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2). Sulphuric Acid And Magnesium Hydroxide Formula.
From www.slideserve.com
PPT Chemical Formulae and Balancing Chemical Equations PowerPoint Sulphuric Acid And Magnesium Hydroxide Formula how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. enter an equation of an ionic chemical equation and press the balance button. if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: The balanced equation will be calculated. Sulphuric Acid And Magnesium Hydroxide Formula.
From infinitylearn.com
Magnesium hydroxide Formula Infinity Learn Sulphuric Acid And Magnesium Hydroxide Formula The solutions involved are colorless. If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.toppr.com
Q5.Write the chemical formula a) Sulphuric acid b) Hydrochloric acid 2 Sulphuric Acid And Magnesium Hydroxide Formula if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. how to balance h2so4 + mg (oh)2 = h2o. Sulphuric Acid And Magnesium Hydroxide Formula.
From w20.b2m.cz
Mg Oh 2 H2so4 EDUCA Sulphuric Acid And Magnesium Hydroxide Formula magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. to determine the formula of the. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.numerade.com
SOLVEDWrite a balanced molecular equation and a net ionic equation for Sulphuric Acid And Magnesium Hydroxide Formula enter an equation of an ionic chemical equation and press the balance button. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. how to balance h2so4 + mg (oh)2 =. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.toppr.com
The equation shows the reaction between magnesium and sulphuric acid Sulphuric Acid And Magnesium Hydroxide Formula to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between. If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other.. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.numerade.com
SOLVED Write the formula for each of the following acids and bases a Sulphuric Acid And Magnesium Hydroxide Formula The balanced equation will be calculated along. The solutions involved are colorless. magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas. Sulphuric Acid And Magnesium Hydroxide Formula.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Sulphuric Acid And Magnesium Hydroxide Formula the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated. Sulphuric Acid And Magnesium Hydroxide Formula.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Sulphuric Acid And Magnesium Hydroxide Formula The balanced equation will be calculated along. if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. enter an equation of an ionic chemical equation and press the. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.echemi.com
What substance is produced when sulphuric acid reacts with both Sulphuric Acid And Magnesium Hydroxide Formula if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: enter an equation of an ionic chemical equation and press the balance button. to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between. the. Sulphuric Acid And Magnesium Hydroxide Formula.
From dayanara-blogmosley.blogspot.com
Magnesium Sulfate and Sodium Carbonate Balanced Equation Sulphuric Acid And Magnesium Hydroxide Formula If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. The solutions involved are colorless. magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. enter an equation of an ionic chemical equation and press the. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.slideshare.net
Acids and Bases Sulphuric Acid And Magnesium Hydroxide Formula If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. to determine the formula of the salt. Sulphuric Acid And Magnesium Hydroxide Formula.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Sulphuric Acid And Magnesium Hydroxide Formula if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. If enough magnesium is used, magnesium sulfate drops out of. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Sulphuric Acid And Magnesium Hydroxide Formula how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. The solutions involved are colorless. magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between. if the base is a metal hydroxide, then. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.advance-africa.com
Chemistry Notes Acid, Bases and Indicators Revision Notes & Tests Sulphuric Acid And Magnesium Hydroxide Formula how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between. enter an equation of an ionic chemical equation and press the balance button. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.tes.com
Ionic Equations Acids and Salts Edexcel 91 Combined Science Teaching Sulphuric Acid And Magnesium Hydroxide Formula enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. The solutions involved are colorless. If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. if the base is a metal hydroxide, then the general formula for the reaction of an acid. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.youtube.com
H2SO4+Mg(OH)2=H2O+MgSO4 Balanced EquationSulphuric Acid+Magnesium Sulphuric Acid And Magnesium Hydroxide Formula magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between. enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. If enough magnesium is used, magnesium sulfate drops. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.tessshebaylo.com
Word Equation For Reaction Between Magnesium And Sulfuric Acid Sulphuric Acid And Magnesium Hydroxide Formula to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between. how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. enter an equation of an ionic chemical equation and press the balance button. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on. Sulphuric Acid And Magnesium Hydroxide Formula.
From fyotbjwgh.blob.core.windows.net
Magnesium Hydroxide Reaction at Robert Adams blog Sulphuric Acid And Magnesium Hydroxide Formula the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. The balanced equation will be calculated along. If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. enter an equation of an ionic chemical equation and press the. Sulphuric Acid And Magnesium Hydroxide Formula.
From exybfixhu.blob.core.windows.net
Magnesium Hydroxide Equation Balanced at Lisa blog Sulphuric Acid And Magnesium Hydroxide Formula how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. The solutions involved are colorless. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. to determine the formula of the salt formed, write the balanced chemical equation for. Sulphuric Acid And Magnesium Hydroxide Formula.
From exyhaniao.blob.core.windows.net
G Magnesium Hydroxide Formula at Mose Newell blog Sulphuric Acid And Magnesium Hydroxide Formula If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between. The balanced equation will be calculated along. how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. The solutions involved. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.youtube.com
How to Write the Formula for Magnesium hydroxide YouTube Sulphuric Acid And Magnesium Hydroxide Formula if the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: The solutions involved are colorless. The balanced equation will be calculated along. to determine the formula of the salt formed, write the balanced chemical equation for the neutralization reaction between. magnesium combined with. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.numerade.com
SOLVED write a balanced chemical equation for the dissociation of Sulphuric Acid And Magnesium Hydroxide Formula enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. If enough magnesium is used, magnesium sulfate drops out of solution to form a white salt. how to balance h2so4 + mg (oh)2 = h2o + mgso4 (sulfuric acid +. if the base is a metal hydroxide,. Sulphuric Acid And Magnesium Hydroxide Formula.
From www.numerade.com
SOLVED What is the correct net ionic equation for the reaction of Sulphuric Acid And Magnesium Hydroxide Formula magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. how. Sulphuric Acid And Magnesium Hydroxide Formula.
From brainly.com
Write a full equation for the reactions between magnesium and sulfuric Sulphuric Acid And Magnesium Hydroxide Formula the chemical equation shows magnesium (mg) and sulfuric acid (h2so4) on one side and magnesium sulfate (mgso4) and hydrogen gas (h2) on the other. enter an equation of an ionic chemical equation and press the balance button. The solutions involved are colorless. magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. to determine the. Sulphuric Acid And Magnesium Hydroxide Formula.