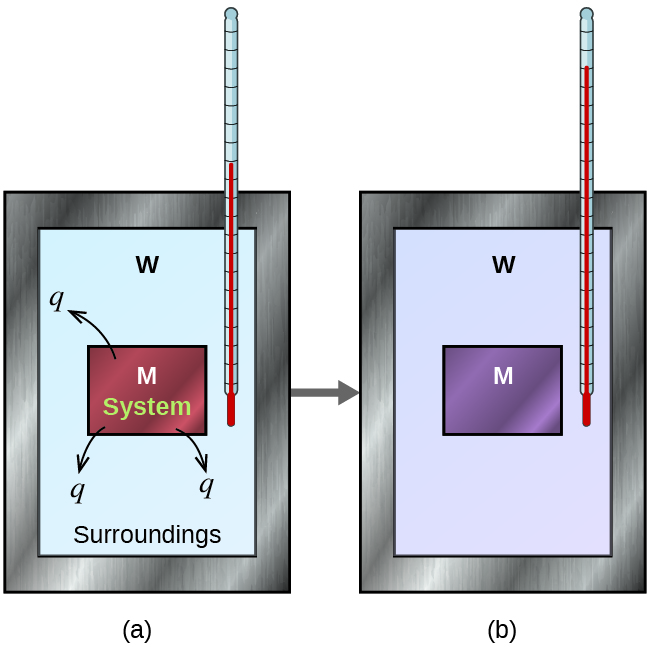
How Accurate Are Calorimeters . Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Explain the technique of calorimetry. Liquid water has one of the highest specific heats known. For example, when an exothermic reaction occurs in solution in a calorimeter, the. By the end of this section, you will be able to: Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. After the calorimeter calibration, the chemist will already have a. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. Calculate and interpret heat and.
from wisc.pb.unizin.org
Liquid water has one of the highest specific heats known. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. After the calorimeter calibration, the chemist will already have a. By the end of this section, you will be able to: Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Calculate and interpret heat and.
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow
How Accurate Are Calorimeters After the calorimeter calibration, the chemist will already have a. For example, when an exothermic reaction occurs in solution in a calorimeter, the. After the calorimeter calibration, the chemist will already have a. Calculate and interpret heat and. By the end of this section, you will be able to: Explain the technique of calorimetry. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Liquid water has one of the highest specific heats known. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument.
From www.animalia-life.club
Calorimeter Diagram How Accurate Are Calorimeters Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. For example, when an exothermic reaction occurs in solution in a calorimeter, the. By the end of this section, you will be able to: Explain the technique of calorimetry. After. How Accurate Are Calorimeters.
From dxoinieui.blob.core.windows.net
Calorimeter Diagram Class 11 at Ollie Stringer blog How Accurate Are Calorimeters By the end of this section, you will be able to: After the calorimeter calibration, the chemist will already have a. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter. How Accurate Are Calorimeters.
From users.highland.edu
Calorimetry How Accurate Are Calorimeters Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. Explain the technique of calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calculate and interpret heat and. After the calorimeter calibration, the chemist will already have a. Liquid water has one of the highest specific heats known. Bomb. How Accurate Are Calorimeters.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 How Accurate Are Calorimeters A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Explain the technique of calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. Calorimetry is used to measure quantities of. How Accurate Are Calorimeters.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 How Accurate Are Calorimeters Calculate and interpret heat and. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical. How Accurate Are Calorimeters.
From www.azom.com
Applications of Tiny Graphite Calorimeters in Clinics How Accurate Are Calorimeters For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Liquid water has one of the highest. How Accurate Are Calorimeters.
From www.chegg.com
Solved Thermometer A bomb calorimeter, or constant volume How Accurate Are Calorimeters Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Calculate and interpret heat and. Explain the technique of calorimetry. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. For example, when an exothermic reaction occurs in solution in a calorimeter, the. After the calorimeter calibration, the chemist will. How Accurate Are Calorimeters.
From www.labster.com
5 Ways to Make Calorimetry and the Bomb Calorimeter More Approachable How Accurate Are Calorimeters By the end of this section, you will be able to: Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Calculate and interpret heat and. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. After the calorimeter calibration, the chemist will already have a. Explain the. How Accurate Are Calorimeters.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson How Accurate Are Calorimeters Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By the end of this section, you will be able to: Calorimetry is used to measure quantities of heat, and can be used to determine the heat. How Accurate Are Calorimeters.
From www.linstitute.net
IB DP Chemistry SL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 How Accurate Are Calorimeters By the end of this section, you will be able to: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Explain the technique of calorimetry. After the calorimeter calibration, the chemist will already have a. Calculate and interpret heat and. Basically, a calorimeter measures the change in temperature of the. How Accurate Are Calorimeters.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts How Accurate Are Calorimeters Calculate and interpret heat and. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Liquid water has one of the highest specific heats known. After the calorimeter calibration, the chemist will already have a. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments.. How Accurate Are Calorimeters.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow How Accurate Are Calorimeters Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Liquid water has one of the highest specific heats known. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. A calorimeter is a device used to measure the amount of heat involved. How Accurate Are Calorimeters.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow How Accurate Are Calorimeters After the calorimeter calibration, the chemist will already have a. Calculate and interpret heat and. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Liquid water has one of the highest specific heats known. Calorimetry is used to measure quantities of heat,. How Accurate Are Calorimeters.
From www.youtube.com
Constructing a Calorimeter YouTube How Accurate Are Calorimeters Explain the technique of calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate. How Accurate Are Calorimeters.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica How Accurate Are Calorimeters Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calculate and interpret heat and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. After the calorimeter calibration, the chemist will already have a. Bomb calorimeters require calibration to. How Accurate Are Calorimeters.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 How Accurate Are Calorimeters Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. Liquid water has one of the highest specific heats known. Basically, a calorimeter measures the change in temperature of the calorimeter and its. How Accurate Are Calorimeters.
From drivenheisenberg.blogspot.com
Which Diagram Is A Bomb Calorimeter Drivenheisenberg How Accurate Are Calorimeters A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Explain the technique of calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Liquid water has one of the highest specific heats known. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and. How Accurate Are Calorimeters.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses How Accurate Are Calorimeters Calculate and interpret heat and. By the end of this section, you will be able to: After the calorimeter calibration, the chemist will already have a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is. How Accurate Are Calorimeters.
From www.animalia-life.club
Calorimeter Diagram How Accurate Are Calorimeters Explain the technique of calorimetry. Liquid water has one of the highest specific heats known. After the calorimeter calibration, the chemist will already have a. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calculate and interpret heat and. Correction for heat. How Accurate Are Calorimeters.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii How Accurate Are Calorimeters Explain the technique of calorimetry. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. After the calorimeter calibration, the chemist will already have a. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. A calorimeter is a device used to measure. How Accurate Are Calorimeters.
From www.slideserve.com
PPT a “ Calorimeter ” PowerPoint Presentation, free download ID7050684 How Accurate Are Calorimeters A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Liquid water has one of the highest specific heats known. For example, when an exothermic reaction occurs in solution in a calorimeter, the. By the end of this section, you will be able to: Explain the technique of calorimetry. Calorimetry is. How Accurate Are Calorimeters.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How Accurate Are Calorimeters By the end of this section, you will be able to: Calculate and interpret heat and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Explain the technique of calorimetry. Calorimetry is used to measure quantities of. How Accurate Are Calorimeters.
From www.pinterest.com.au
Pin on Science Friday How Accurate Are Calorimeters By the end of this section, you will be able to: Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Liquid water has one of the highest specific heats known. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. After the calorimeter calibration,. How Accurate Are Calorimeters.
From www.animalia-life.club
Food Calorimeter How Accurate Are Calorimeters Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Liquid water has one of the highest specific heats known. After the calorimeter calibration, the chemist will already have a. Calculate and interpret heat and. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Calorimetry is used to measure. How Accurate Are Calorimeters.
From www.youtube.com
050 Calorimetry YouTube How Accurate Are Calorimeters Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. For example, when an exothermic reaction occurs in solution in a calorimeter, the. After the calorimeter calibration, the chemist will already have a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By the. How Accurate Are Calorimeters.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation How Accurate Are Calorimeters By the end of this section, you will be able to: Liquid water has one of the highest specific heats known. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Explain the technique of calorimetry. After the calorimeter calibration, the chemist will already have a. Basically, a calorimeter. How Accurate Are Calorimeters.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts How Accurate Are Calorimeters Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Liquid water has one of the highest specific heats known. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. How Accurate Are Calorimeters.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube How Accurate Are Calorimeters Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Liquid water has one of the highest specific heats known. A calorimeter. How Accurate Are Calorimeters.
From philschatz.com
Calorimetry · Chemistry How Accurate Are Calorimeters Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Explain the technique of calorimetry. By the end of this section, you will be able to: Calculate and interpret heat and. A calorimeter is a. How Accurate Are Calorimeters.
From guernseydonkey.com
How is the Caloric Value of Food Measured? How Accurate Are Calorimeters Explain the technique of calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Liquid water has one of the highest specific heats. How Accurate Are Calorimeters.
From www.scienceabc.com
How Do We Calculate How Many Carbs Are In The Food? How Accurate Are Calorimeters Calculate and interpret heat and. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. By the end of this section, you will be able to: Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure. How Accurate Are Calorimeters.
From www.thoughtco.com
Calorimeter Definition in Chemistry How Accurate Are Calorimeters A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Liquid water has one of the highest specific heats known. Explain the technique of calorimetry. After the calorimeter calibration, the chemist will already have a. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument.. How Accurate Are Calorimeters.
From klayjstec.blob.core.windows.net
How Calorimeter Function at Geneva Katzman blog How Accurate Are Calorimeters By the end of this section, you will be able to: After the calorimeter calibration, the chemist will already have a. Explain the technique of calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. Calculate and interpret heat and.. How Accurate Are Calorimeters.
From joiksvxlu.blob.core.windows.net
How Does Calorimeter Constant Work at Stacy Cavallo blog How Accurate Are Calorimeters A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Liquid water has one of the highest specific heats known. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Calorimetry is used to measure quantities of heat, and can be used to determine the. How Accurate Are Calorimeters.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure How Accurate Are Calorimeters Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Correction for heat exchange with the surroundings in heat conduction and power compensation calorimeters is instrument. After the calorimeter calibration, the chemist will already have a. Liquid water has one of the highest specific heats known. For example, when an exothermic reaction occurs in solution in. How Accurate Are Calorimeters.