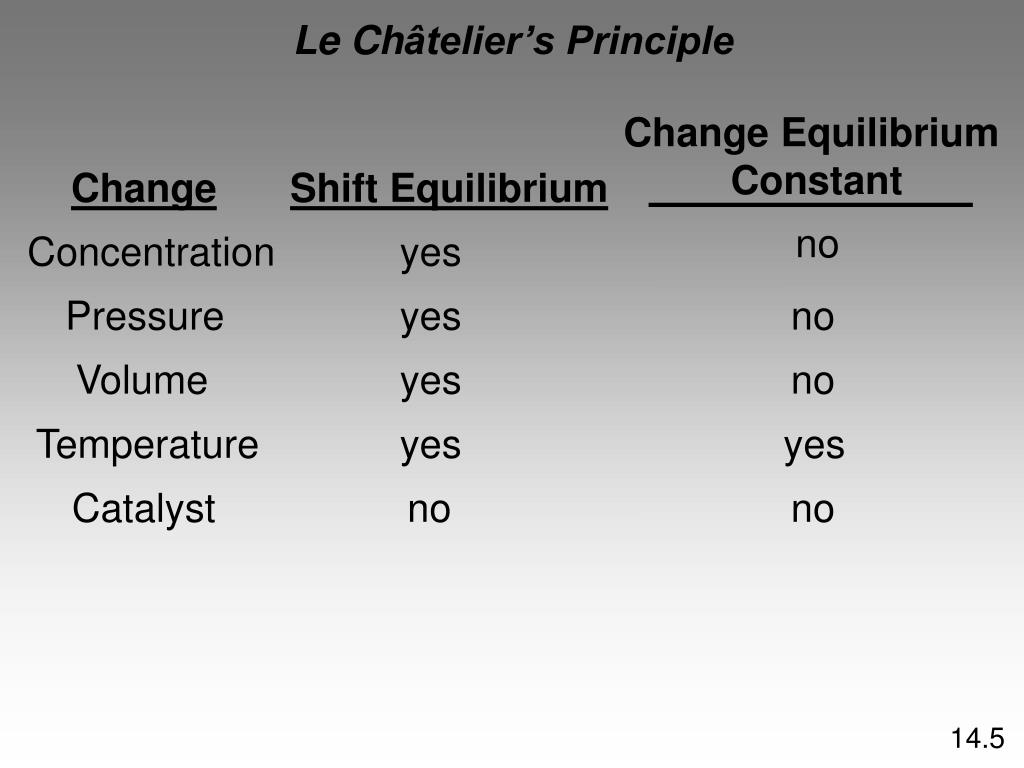
A Catalyst Does Not Change The Value Of The Rate Constant . The numerical value of k, however, does not change as the reaction progresses under a given set of conditions. If the rate constant doubles, for example,. It assumes familiarity with basic concepts in the collision. That is, a catalyst does not change the fundamental equilibrium (or the underlying thermodynamics) of a reaction. Changes in the rate constant or of the activation energy are obvious ways of measuring the efficacy of a catalyst. This is shown mathematically in the arrhenius equation. But two other terms have come into use that have. A catalyst always increases the rate of a reaction; This page describes and explains the way that adding a catalyst affects the rate of a reaction. If you change the temperature or the catalyst, for example, the rate constant changes. This page explains how adding a catalyst affects the rate of a reaction. Any substance that reduces the rate of a reaction is called an inhibitor. It has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well as the forward reaction to attain equilibrium fast. It assumes that you are already familiar with basic ideas about the collision theory of reaction.
from www.slideserve.com
If the rate constant doubles, for example,. A catalyst always increases the rate of a reaction; It assumes familiarity with basic concepts in the collision. The numerical value of k, however, does not change as the reaction progresses under a given set of conditions. If you change the temperature or the catalyst, for example, the rate constant changes. This page explains how adding a catalyst affects the rate of a reaction. This is shown mathematically in the arrhenius equation. Any substance that reduces the rate of a reaction is called an inhibitor. Changes in the rate constant or of the activation energy are obvious ways of measuring the efficacy of a catalyst. But two other terms have come into use that have.
PPT Chapter 18 Rates of Reaction PowerPoint Presentation, free
A Catalyst Does Not Change The Value Of The Rate Constant This page explains how adding a catalyst affects the rate of a reaction. A catalyst always increases the rate of a reaction; It assumes familiarity with basic concepts in the collision. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well as the forward reaction to attain equilibrium fast. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This is shown mathematically in the arrhenius equation. Changes in the rate constant or of the activation energy are obvious ways of measuring the efficacy of a catalyst. The numerical value of k, however, does not change as the reaction progresses under a given set of conditions. That is, a catalyst does not change the fundamental equilibrium (or the underlying thermodynamics) of a reaction. If you change the temperature or the catalyst, for example, the rate constant changes. Any substance that reduces the rate of a reaction is called an inhibitor. If the rate constant doubles, for example,. But two other terms have come into use that have.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation A Catalyst Does Not Change The Value Of The Rate Constant That is, a catalyst does not change the fundamental equilibrium (or the underlying thermodynamics) of a reaction. The numerical value of k, however, does not change as the reaction progresses under a given set of conditions. A catalyst always increases the rate of a reaction; It assumes familiarity with basic concepts in the collision. It has been observed that a. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction A Catalyst Does Not Change The Value Of The Rate Constant If you change the temperature or the catalyst, for example, the rate constant changes. This page explains how adding a catalyst affects the rate of a reaction. A catalyst always increases the rate of a reaction; It assumes familiarity with basic concepts in the collision. Any substance that reduces the rate of a reaction is called an inhibitor. That is,. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.numerade.com
SOLVED A catalyst in a chemical reaction (a) Decreases rate constant A Catalyst Does Not Change The Value Of The Rate Constant This page explains how adding a catalyst affects the rate of a reaction. Any substance that reduces the rate of a reaction is called an inhibitor. If you change the temperature or the catalyst, for example, the rate constant changes. But two other terms have come into use that have. This page describes and explains the way that adding a. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.doubtnut.com
Addition of catalyst does not change K(P) but changes DeltaH A Catalyst Does Not Change The Value Of The Rate Constant It assumes familiarity with basic concepts in the collision. If you change the temperature or the catalyst, for example, the rate constant changes. This is shown mathematically in the arrhenius equation. If the rate constant doubles, for example,. It has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.doubtnut.com
Explain with the help of potential energy barrier how does a catalys A Catalyst Does Not Change The Value Of The Rate Constant Any substance that reduces the rate of a reaction is called an inhibitor. If you change the temperature or the catalyst, for example, the rate constant changes. It has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well as the forward reaction to attain equilibrium fast. That is, a. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.learnerslodge.com.sg
UNDERSTANDING H2 CHEMISTRY EQUILIBRIA A Catalyst Does Not Change The Value Of The Rate Constant If you change the temperature or the catalyst, for example, the rate constant changes. This is shown mathematically in the arrhenius equation. That is, a catalyst does not change the fundamental equilibrium (or the underlying thermodynamics) of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. If the rate constant doubles,. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.numerade.com
SOLVEDWhich of the following statement(s) is(are) true? a. The half A Catalyst Does Not Change The Value Of The Rate Constant That is, a catalyst does not change the fundamental equilibrium (or the underlying thermodynamics) of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.slideserve.com
PPT Le Châtelier’s Principle PowerPoint Presentation, free download A Catalyst Does Not Change The Value Of The Rate Constant Changes in the rate constant or of the activation energy are obvious ways of measuring the efficacy of a catalyst. That is, a catalyst does not change the fundamental equilibrium (or the underlying thermodynamics) of a reaction. If you change the temperature or the catalyst, for example, the rate constant changes. This page explains how adding a catalyst affects the. A Catalyst Does Not Change The Value Of The Rate Constant.
From slideplayer.com
Applications of Equilibrium Constants ppt download A Catalyst Does Not Change The Value Of The Rate Constant If you change the temperature or the catalyst, for example, the rate constant changes. It has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well as the forward reaction to attain equilibrium fast. But two other terms have come into use that have. That is, a catalyst does not. A Catalyst Does Not Change The Value Of The Rate Constant.
From gioqbjigg.blob.core.windows.net
Catalyst Does Not Change The Reaction Mechanism at Yvette Smith blog A Catalyst Does Not Change The Value Of The Rate Constant If you change the temperature or the catalyst, for example, the rate constant changes. It has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well as the forward reaction to attain equilibrium fast. The numerical value of k, however, does not change as the reaction progresses under a given. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.slideserve.com
PPT C3 Contents PowerPoint Presentation, free download ID1586729 A Catalyst Does Not Change The Value Of The Rate Constant That is, a catalyst does not change the fundamental equilibrium (or the underlying thermodynamics) of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst always increases the rate of a reaction; Any substance that reduces the rate of a reaction is called an inhibitor. But two other terms. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.slideserve.com
PPT Chapter 18 Rates of Reaction PowerPoint Presentation, free A Catalyst Does Not Change The Value Of The Rate Constant This page describes and explains the way that adding a catalyst affects the rate of a reaction. It has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well as the forward reaction to attain equilibrium fast. This is shown mathematically in the arrhenius equation. That is, a catalyst does. A Catalyst Does Not Change The Value Of The Rate Constant.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis A Catalyst Does Not Change The Value Of The Rate Constant The numerical value of k, however, does not change as the reaction progresses under a given set of conditions. This is shown mathematically in the arrhenius equation. Changes in the rate constant or of the activation energy are obvious ways of measuring the efficacy of a catalyst. A catalyst always increases the rate of a reaction; This page explains how. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.doubtnut.com
A catalyst does not change the state of equlibrium in a chemic A Catalyst Does Not Change The Value Of The Rate Constant It assumes familiarity with basic concepts in the collision. Any substance that reduces the rate of a reaction is called an inhibitor. This is shown mathematically in the arrhenius equation. It has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well as the forward reaction to attain equilibrium fast.. A Catalyst Does Not Change The Value Of The Rate Constant.
From slideplayer.com
Catalyst ppt download A Catalyst Does Not Change The Value Of The Rate Constant It has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well as the forward reaction to attain equilibrium fast. This is shown mathematically in the arrhenius equation. But two other terms have come into use that have. This page describes and explains the way that adding a catalyst affects. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the A Catalyst Does Not Change The Value Of The Rate Constant This page describes and explains the way that adding a catalyst affects the rate of a reaction. Any substance that reduces the rate of a reaction is called an inhibitor. If the rate constant doubles, for example,. If you change the temperature or the catalyst, for example, the rate constant changes. This page explains how adding a catalyst affects the. A Catalyst Does Not Change The Value Of The Rate Constant.
From slideplayer.com
Chapter 15 Chemical Equilibrium occurs when opposing reactions proceed A Catalyst Does Not Change The Value Of The Rate Constant A catalyst always increases the rate of a reaction; It assumes familiarity with basic concepts in the collision. Any substance that reduces the rate of a reaction is called an inhibitor. It assumes that you are already familiar with basic ideas about the collision theory of reaction. If you change the temperature or the catalyst, for example, the rate constant. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.slideserve.com
PPT Chapter 13 Equilibrium PowerPoint Presentation, free download A Catalyst Does Not Change The Value Of The Rate Constant That is, a catalyst does not change the fundamental equilibrium (or the underlying thermodynamics) of a reaction. Changes in the rate constant or of the activation energy are obvious ways of measuring the efficacy of a catalyst. It assumes that you are already familiar with basic ideas about the collision theory of reaction. If you change the temperature or the. A Catalyst Does Not Change The Value Of The Rate Constant.
From askfilo.com
Given below are two statements StatementI Catalyst does not change eq.. A Catalyst Does Not Change The Value Of The Rate Constant If you change the temperature or the catalyst, for example, the rate constant changes. Any substance that reduces the rate of a reaction is called an inhibitor. This is shown mathematically in the arrhenius equation. It has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well as the forward. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.numerade.com
SOLVEDA catalyst in a chemical reaction (a) decreases rate constant of A Catalyst Does Not Change The Value Of The Rate Constant The numerical value of k, however, does not change as the reaction progresses under a given set of conditions. This page explains how adding a catalyst affects the rate of a reaction. Any substance that reduces the rate of a reaction is called an inhibitor. If the rate constant doubles, for example,. Changes in the rate constant or of the. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.doubtnut.com
Addition of catalyst does not change K(p) but changes DeltaH. A Catalyst Does Not Change The Value Of The Rate Constant If you change the temperature or the catalyst, for example, the rate constant changes. It has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well as the forward reaction to attain equilibrium fast. A catalyst always increases the rate of a reaction; This is shown mathematically in the arrhenius. A Catalyst Does Not Change The Value Of The Rate Constant.
From slideplayer.com
Chemical Equilibrium Chapter ppt download A Catalyst Does Not Change The Value Of The Rate Constant This is shown mathematically in the arrhenius equation. This page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Changes in the rate constant or of the activation energy are obvious ways of measuring the efficacy of a catalyst. It assumes familiarity. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial A Catalyst Does Not Change The Value Of The Rate Constant It assumes familiarity with basic concepts in the collision. It assumes that you are already familiar with basic ideas about the collision theory of reaction. A catalyst always increases the rate of a reaction; But two other terms have come into use that have. This page describes and explains the way that adding a catalyst affects the rate of a. A Catalyst Does Not Change The Value Of The Rate Constant.
From slideplayer.com
Equilibrium. ppt download A Catalyst Does Not Change The Value Of The Rate Constant It assumes that you are already familiar with basic ideas about the collision theory of reaction. If the rate constant doubles, for example,. This is shown mathematically in the arrhenius equation. This page explains how adding a catalyst affects the rate of a reaction. The numerical value of k, however, does not change as the reaction progresses under a given. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID A Catalyst Does Not Change The Value Of The Rate Constant The numerical value of k, however, does not change as the reaction progresses under a given set of conditions. If the rate constant doubles, for example,. It assumes that you are already familiar with basic ideas about the collision theory of reaction. It assumes familiarity with basic concepts in the collision. But two other terms have come into use that. A Catalyst Does Not Change The Value Of The Rate Constant.
From ppt-online.org
Rates of reaction презентация онлайн A Catalyst Does Not Change The Value Of The Rate Constant This page explains how adding a catalyst affects the rate of a reaction. If the rate constant doubles, for example,. Any substance that reduces the rate of a reaction is called an inhibitor. But two other terms have come into use that have. It has been observed that a catalyst does not change the equilibrium constant for a reaction but. A Catalyst Does Not Change The Value Of The Rate Constant.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General A Catalyst Does Not Change The Value Of The Rate Constant If the rate constant doubles, for example,. It assumes familiarity with basic concepts in the collision. It assumes that you are already familiar with basic ideas about the collision theory of reaction. But two other terms have come into use that have. Any substance that reduces the rate of a reaction is called an inhibitor. This page describes and explains. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID A Catalyst Does Not Change The Value Of The Rate Constant A catalyst always increases the rate of a reaction; The numerical value of k, however, does not change as the reaction progresses under a given set of conditions. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision. That is, a catalyst does not. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and A Catalyst Does Not Change The Value Of The Rate Constant If you change the temperature or the catalyst, for example, the rate constant changes. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. It has been observed that a catalyst does not change the equilibrium constant for a reaction. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.chegg.com
Solved Which is true about rates and catalysts? The rate A Catalyst Does Not Change The Value Of The Rate Constant But two other terms have come into use that have. This is shown mathematically in the arrhenius equation. The numerical value of k, however, does not change as the reaction progresses under a given set of conditions. Changes in the rate constant or of the activation energy are obvious ways of measuring the efficacy of a catalyst. A catalyst always. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review A Catalyst Does Not Change The Value Of The Rate Constant If the rate constant doubles, for example,. Any substance that reduces the rate of a reaction is called an inhibitor. A catalyst always increases the rate of a reaction; This is shown mathematically in the arrhenius equation. The numerical value of k, however, does not change as the reaction progresses under a given set of conditions. That is, a catalyst. A Catalyst Does Not Change The Value Of The Rate Constant.
From byjus.com
Does the value of rate constant change due to catayst A Catalyst Does Not Change The Value Of The Rate Constant But two other terms have come into use that have. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Any substance that reduces the rate of a reaction is called an inhibitor. This is shown mathematically in the arrhenius. A Catalyst Does Not Change The Value Of The Rate Constant.
From slideplayer.com
Chemical Equilibrium Chapter ppt download A Catalyst Does Not Change The Value Of The Rate Constant This is shown mathematically in the arrhenius equation. That is, a catalyst does not change the fundamental equilibrium (or the underlying thermodynamics) of a reaction. But two other terms have come into use that have. It has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well as the forward. A Catalyst Does Not Change The Value Of The Rate Constant.
From slideplayer.com
Factors That Affect Equilibrium ppt download A Catalyst Does Not Change The Value Of The Rate Constant The numerical value of k, however, does not change as the reaction progresses under a given set of conditions. Any substance that reduces the rate of a reaction is called an inhibitor. That is, a catalyst does not change the fundamental equilibrium (or the underlying thermodynamics) of a reaction. If you change the temperature or the catalyst, for example, the. A Catalyst Does Not Change The Value Of The Rate Constant.
From www.slideserve.com
PPT Chapter 18 Rates of Reaction PowerPoint Presentation, free A Catalyst Does Not Change The Value Of The Rate Constant It assumes that you are already familiar with basic ideas about the collision theory of reaction. This is shown mathematically in the arrhenius equation. Changes in the rate constant or of the activation energy are obvious ways of measuring the efficacy of a catalyst. If the rate constant doubles, for example,. This page describes and explains the way that adding. A Catalyst Does Not Change The Value Of The Rate Constant.