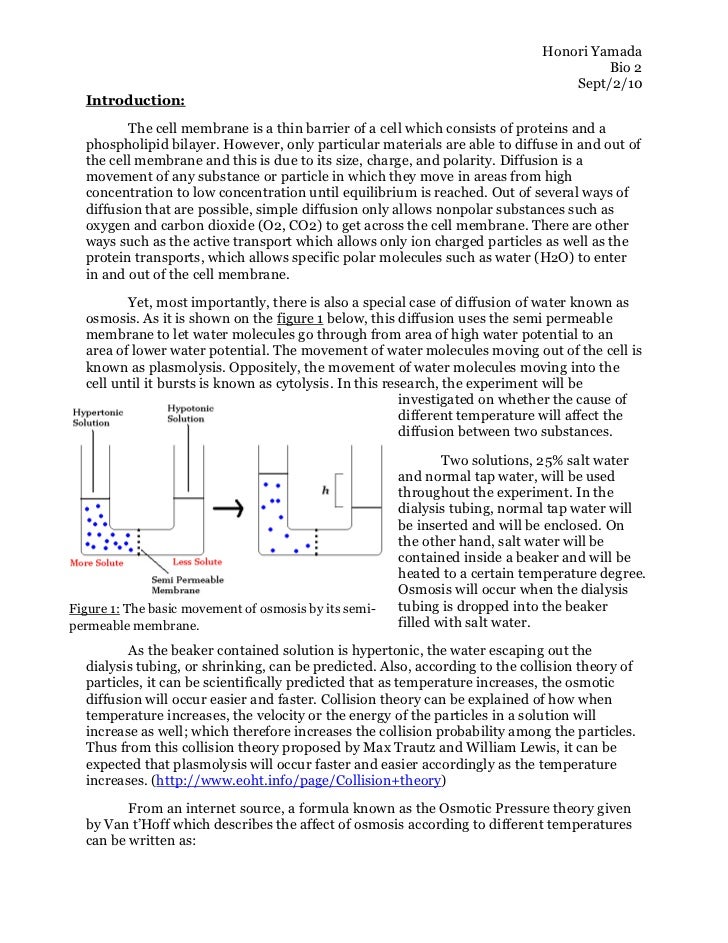
Potato Salt Water Osmosis Lab Report Discussion . A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. This leads to shrinking of the potato cells which explains why the potato. Because there are no salts in. This material is based upon. To demonstrate the concept of osmosis using potatoes and fruit. Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium. In this lab, we will be researching the effects of saltwater on pieces of potato. Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. Exploring osmotic processes in potatoes: Effects of salt concentration on mass. We will explore and observe the. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution.
from jetpaper.web.fc2.com
This leads to shrinking of the potato cells which explains why the potato. Because there are no salts in. A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. In this lab, we will be researching the effects of saltwater on pieces of potato. Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. This material is based upon. Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium. Exploring osmotic processes in potatoes: We will explore and observe the.
lab report on osmosis and diffusion/
Potato Salt Water Osmosis Lab Report Discussion In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. To demonstrate the concept of osmosis using potatoes and fruit. Effects of salt concentration on mass. Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. In this lab, we will be researching the effects of saltwater on pieces of potato. We will explore and observe the. A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. Because there are no salts in. This leads to shrinking of the potato cells which explains why the potato. Exploring osmotic processes in potatoes: This material is based upon. Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium.
From classschoolhybridises.z21.web.core.windows.net
Discussion For Osmosis In Potato Lab Potato Salt Water Osmosis Lab Report Discussion In this lab, we will be researching the effects of saltwater on pieces of potato. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. A process called. Potato Salt Water Osmosis Lab Report Discussion.
From www.youtube.com
Potato Osmosis Experiment + Steps. YouTube Potato Salt Water Osmosis Lab Report Discussion In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. This material is based upon. Exploring osmotic processes in potatoes: This leads to shrinking of the potato cells which explains why the potato. To demonstrate the concept of osmosis using potatoes and fruit. In this lab, we will be researching the. Potato Salt Water Osmosis Lab Report Discussion.
From about.dataclassroom.com
Potato Osmosis Lab — DataClassroom Potato Salt Water Osmosis Lab Report Discussion Exploring osmotic processes in potatoes: This leads to shrinking of the potato cells which explains why the potato. A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. Effects of salt concentration on mass. Because there are no salts in. In this experiment you will see the effects of putting. Potato Salt Water Osmosis Lab Report Discussion.
From webapi.bu.edu
🏆 Potato osmosis lab. Potato Osmosis Lab. 20221018 Potato Salt Water Osmosis Lab Report Discussion In this lab, we will be researching the effects of saltwater on pieces of potato. Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. Because there are no salts in. A process called osmosis helps the water move from the soil into the plant roots—and then. Potato Salt Water Osmosis Lab Report Discussion.
From childhealthpolicy.vumc.org
💌 Potato osmosis lab. Potato (Osmosis) Experiment. 20221101 Potato Salt Water Osmosis Lab Report Discussion This material is based upon. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. In this lab, we will be researching the effects of saltwater on pieces of potato. Exploring osmotic processes in potatoes: Once the salt concentration in the cup gets higher than inside the potato cells, water moves. Potato Salt Water Osmosis Lab Report Discussion.
From www.scribd.com
Potato Lab Report Osmosis Chemical Substances Free 30day Trial Potato Salt Water Osmosis Lab Report Discussion A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. This leads to shrinking of the potato cells which explains why the potato. We will explore and observe the. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. Exploring osmotic. Potato Salt Water Osmosis Lab Report Discussion.
From discountpapers.web.fc2.com
potato osmolarity lab report Potato Salt Water Osmosis Lab Report Discussion Because there are no salts in. To demonstrate the concept of osmosis using potatoes and fruit. A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. This leads to shrinking of the potato cells which explains why the potato. In this lab, we will be researching the effects of saltwater. Potato Salt Water Osmosis Lab Report Discussion.
From classlibvera.z19.web.core.windows.net
Method For Osmosis Potato Lab Report Potato Salt Water Osmosis Lab Report Discussion This leads to shrinking of the potato cells which explains why the potato. We will explore and observe the. Effects of salt concentration on mass. Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. In this experiment you will see the effects of putting a potato. Potato Salt Water Osmosis Lab Report Discussion.
From www.thinkswap.com
Osmosis in Potatoes Biology Year 12 SACE Thinkswap Potato Salt Water Osmosis Lab Report Discussion To demonstrate the concept of osmosis using potatoes and fruit. This material is based upon. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. In this lab, we will be researching the effects of saltwater on pieces of potato. We will explore and observe the. Because there are no salts. Potato Salt Water Osmosis Lab Report Discussion.
From about.dataclassroom.com
Potato Osmosis Lab — DataClassroom Potato Salt Water Osmosis Lab Report Discussion To demonstrate the concept of osmosis using potatoes and fruit. Exploring osmotic processes in potatoes: This material is based upon. Because there are no salts in. In this lab, we will be researching the effects of saltwater on pieces of potato. We will explore and observe the. Once the salt concentration in the cup gets higher than inside the potato. Potato Salt Water Osmosis Lab Report Discussion.
From ar.inspiredpencil.com
Osmosis In Potatoes Lab Report Potato Salt Water Osmosis Lab Report Discussion Because there are no salts in. A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. To demonstrate the concept of osmosis using potatoes and fruit. Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium. Once the salt concentration in the cup gets higher. Potato Salt Water Osmosis Lab Report Discussion.
From tukioka-clinic.com
👍 Osmosis potato experiment results. Osmosis lab report. 20190131 Potato Salt Water Osmosis Lab Report Discussion Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium. In this lab, we will be researching the effects of saltwater on pieces of potato. A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. In this experiment you will see the effects of putting. Potato Salt Water Osmosis Lab Report Discussion.
From cupsoguepictures.com
️ Potato osmosis lab conclusion. Osmosis Experiment For Kids Potato Potato Salt Water Osmosis Lab Report Discussion This material is based upon. In this lab, we will be researching the effects of saltwater on pieces of potato. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. To demonstrate the concept of osmosis using potatoes and fruit. We will explore and observe the. Osmosis refers to the movement. Potato Salt Water Osmosis Lab Report Discussion.
From paperap.com
Experiment with potato and salt water Free Essay Example Potato Salt Water Osmosis Lab Report Discussion To demonstrate the concept of osmosis using potatoes and fruit. We will explore and observe the. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. Exploring osmotic. Potato Salt Water Osmosis Lab Report Discussion.
From www.slideshare.net
Osmosis lab of potato in three types of water Potato Salt Water Osmosis Lab Report Discussion To demonstrate the concept of osmosis using potatoes and fruit. Because there are no salts in. Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. This material is based upon. In this lab, we will be researching the effects of saltwater on pieces of potato. Osmosis. Potato Salt Water Osmosis Lab Report Discussion.
From studylib.net
Plasmolysis and Osmosis Lab with Potatoes Potato Salt Water Osmosis Lab Report Discussion Because there are no salts in. Exploring osmotic processes in potatoes: In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. We will explore and observe the. Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. This. Potato Salt Water Osmosis Lab Report Discussion.
From pt.scribd.com
Osmosis Potato Lab Osmosis Experiment Potato Salt Water Osmosis Lab Report Discussion This leads to shrinking of the potato cells which explains why the potato. Because there are no salts in. This material is based upon. Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. Effects of salt concentration on mass. Exploring osmotic processes in potatoes: In this. Potato Salt Water Osmosis Lab Report Discussion.
From worksheetlibray.z13.web.core.windows.net
Lab Report On Osmosis Using Potatoes Potato Salt Water Osmosis Lab Report Discussion In this lab, we will be researching the effects of saltwater on pieces of potato. This leads to shrinking of the potato cells which explains why the potato. Because there are no salts in. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. We will explore and observe the. To. Potato Salt Water Osmosis Lab Report Discussion.
From nerdyseal.com
Potato osmosis lab report 522 Words NerdySeal Potato Salt Water Osmosis Lab Report Discussion Exploring osmotic processes in potatoes: Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium. This leads to shrinking of the potato cells which explains why the potato. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. To demonstrate the concept of osmosis using potatoes. Potato Salt Water Osmosis Lab Report Discussion.
From www.edrawmax.com
Potato Osmosis Lab Report EdrawMax EdrawMax Templates Potato Salt Water Osmosis Lab Report Discussion Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. We will explore and observe the. Because there are no salts in. To demonstrate the concept of osmosis using potatoes and fruit. Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium.. Potato Salt Water Osmosis Lab Report Discussion.
From www.thinkswap.com
Effect of salt concentration on osmosis in potato cells Biology Potato Salt Water Osmosis Lab Report Discussion We will explore and observe the. This leads to shrinking of the potato cells which explains why the potato. A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. In this lab, we will be researching the effects of saltwater on pieces of potato. Osmosis refers to the movement of. Potato Salt Water Osmosis Lab Report Discussion.
From paperap.com
Potato Salt Water Osmosis Lab Report Free Essay Example Potato Salt Water Osmosis Lab Report Discussion Because there are no salts in. A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. To demonstrate the concept of osmosis using potatoes and fruit. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. Once the salt concentration in. Potato Salt Water Osmosis Lab Report Discussion.
From studylibrarybeverly.z21.web.core.windows.net
Osmosis Lab Report Potato Potato Salt Water Osmosis Lab Report Discussion This leads to shrinking of the potato cells which explains why the potato. To demonstrate the concept of osmosis using potatoes and fruit. Because there are no salts in. We will explore and observe the. A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. In this experiment you will. Potato Salt Water Osmosis Lab Report Discussion.
From animalia-life.club
Osmosis In Potatoes Diagram Potato Salt Water Osmosis Lab Report Discussion We will explore and observe the. This leads to shrinking of the potato cells which explains why the potato. Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium. Because there are no salts in. In this lab, we will be researching the effects of saltwater on pieces of potato. Once the salt concentration in. Potato Salt Water Osmosis Lab Report Discussion.
From paperap.com
Osmosis Potato Experiment Control Variables Free Essay Example Potato Salt Water Osmosis Lab Report Discussion In this lab, we will be researching the effects of saltwater on pieces of potato. Effects of salt concentration on mass. Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium. Exploring osmotic processes in potatoes: A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's. Potato Salt Water Osmosis Lab Report Discussion.
From webapi.bu.edu
Potato osmosis lab results. Sample Lab Report. 20221027 Potato Salt Water Osmosis Lab Report Discussion This leads to shrinking of the potato cells which explains why the potato. This material is based upon. Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. We will explore and observe the. Effects of. Potato Salt Water Osmosis Lab Report Discussion.
From jetpaper.web.fc2.com
lab report on osmosis and diffusion/ Potato Salt Water Osmosis Lab Report Discussion A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium. Effects of salt concentration on mass. This leads to shrinking of the potato cells which explains why the potato. We will explore and observe the.. Potato Salt Water Osmosis Lab Report Discussion.
From es.scribd.com
lab report osmosis in a potato Osmosis Experiment Prueba gratuita Potato Salt Water Osmosis Lab Report Discussion To demonstrate the concept of osmosis using potatoes and fruit. We will explore and observe the. A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. Effects of salt concentration on mass. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic. Potato Salt Water Osmosis Lab Report Discussion.
From www.studocu.com
Osmosis in potatoes experiment plan Osmosis in Potatoes my Potato Salt Water Osmosis Lab Report Discussion In this lab, we will be researching the effects of saltwater on pieces of potato. To demonstrate the concept of osmosis using potatoes and fruit. We will explore and observe the. Effects of salt concentration on mass. Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium. This material is based upon. A process called. Potato Salt Water Osmosis Lab Report Discussion.
From funscienceactivityclub.blogspot.com
Osmosis in potato Potato Salt Water Osmosis Lab Report Discussion Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium. This leads to shrinking of the potato cells which explains why the potato. We will explore and observe the. In this lab, we will be researching the effects of saltwater on pieces of potato. Effects of salt concentration on mass. This material is based upon.. Potato Salt Water Osmosis Lab Report Discussion.
From studylib.net
Lab Report Potato Osmosis Potato Salt Water Osmosis Lab Report Discussion Osmosis refers to the movement of water molecules across a membrane trying to achieve equilibrium. This material is based upon. Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. In this lab, we will be researching the effects of saltwater on pieces of potato. To demonstrate. Potato Salt Water Osmosis Lab Report Discussion.
From lemurianembassy.com
️ Potato osmosis lab report conclusion. AP Biology Lab Osmosis and Potato Salt Water Osmosis Lab Report Discussion Once the salt concentration in the cup gets higher than inside the potato cells, water moves out of the potato into the cup. In this lab, we will be researching the effects of saltwater on pieces of potato. To demonstrate the concept of osmosis using potatoes and fruit. This leads to shrinking of the potato cells which explains why the. Potato Salt Water Osmosis Lab Report Discussion.
From www.slideshare.net
Osmosis lab of potato in three types of water Potato Salt Water Osmosis Lab Report Discussion A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. To demonstrate the concept of osmosis using potatoes and fruit. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. In this lab, we will be researching the effects of saltwater. Potato Salt Water Osmosis Lab Report Discussion.
From www.scribd.com
Sample Lab Report Potato Osmosis Potato Salt Water Osmosis Lab Report Discussion Effects of salt concentration on mass. Because there are no salts in. In this lab, we will be researching the effects of saltwater on pieces of potato. This material is based upon. In this experiment you will see the effects of putting a potato in a hypertonic solution and hypotonic solution. Once the salt concentration in the cup gets higher. Potato Salt Water Osmosis Lab Report Discussion.
From printablezoneungear.z21.web.core.windows.net
Discussion For Osmosis In Potato Lab Potato Salt Water Osmosis Lab Report Discussion We will explore and observe the. Because there are no salts in. In this lab, we will be researching the effects of saltwater on pieces of potato. Exploring osmotic processes in potatoes: Effects of salt concentration on mass. A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. To demonstrate. Potato Salt Water Osmosis Lab Report Discussion.