Does An Atom Have Volume . A convenient unit of length for measuring. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy. It is now known that an atom has a positively charged nucleus that makes up more than 99.9% of the atom’s mass but only about 1/100,000 of its volume. Atoms are incredibly small, typically around 100 picometers in diameter. The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. The nucleus is composed of. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier.
from www.sciencefacts.net
For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. The nucleus is composed of. Atoms are incredibly small, typically around 100 picometers in diameter. A convenient unit of length for measuring. The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. It is now known that an atom has a positively charged nucleus that makes up more than 99.9% of the atom’s mass but only about 1/100,000 of its volume. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to.
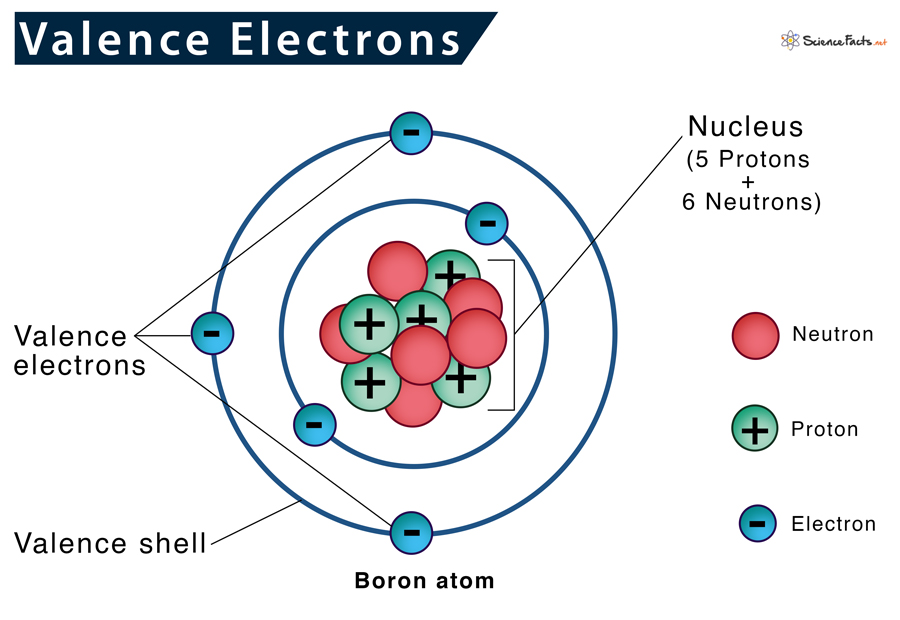
Valence Electrons Definition, Location, Importance, and Diagram
Does An Atom Have Volume It is now known that an atom has a positively charged nucleus that makes up more than 99.9% of the atom’s mass but only about 1/100,000 of its volume. The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. Atoms are incredibly small, typically around 100 picometers in diameter. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. It is now known that an atom has a positively charged nucleus that makes up more than 99.9% of the atom’s mass but only about 1/100,000 of its volume. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy. For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. A convenient unit of length for measuring. The nucleus is composed of.
From www.expii.com
Atoms — Definition & Overview Expii Does An Atom Have Volume The nucleus is composed of. Atoms are incredibly small, typically around 100 picometers in diameter. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. The nucleus contains the. Does An Atom Have Volume.
From solbergsphyscience.wikispaces.com
solbergsphyscience The Structure of the Atom Does An Atom Have Volume About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy. Atoms are incredibly small, typically around 100 picometers in diameter. It is now known that an atom has a positively. Does An Atom Have Volume.
From spmscience.blog.onlinetuition.com.my
4.2 Structure of Atoms SPM Science Does An Atom Have Volume About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. The nucleus contains the majority of an. Does An Atom Have Volume.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Does An Atom Have Volume It is now known that an atom has a positively charged nucleus that makes up more than 99.9% of the atom’s mass but only about 1/100,000 of its volume. The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. About 99.94% of an atom’s mass is in its nucleus, with protons and. Does An Atom Have Volume.
From learninginhand.com
Label Parts of an Atom — Learning in Hand with Tony Vincent Does An Atom Have Volume Atoms are incredibly small, typically around 100 picometers in diameter. A convenient unit of length for measuring. For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. The nucleus contains the majority. Does An Atom Have Volume.
From www.youtube.com
CHM122 2_6_1 Volume of an Atom YouTube Does An Atom Have Volume The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. About 99.94% of an. Does An Atom Have Volume.
From www.thoughtco.com
The Definition of Atomic Volume and How to Calculate It Does An Atom Have Volume The nucleus is composed of. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. The nucleus contains the majority of an atom’s mass because protons and neutrons are. Does An Atom Have Volume.
From www.thoughtco.com
Basic Model of the Atom Atomic Theory Does An Atom Have Volume For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. The nucleus is composed of. It is now known that an atom has a positively charged nucleus that makes up more than 99.9% of. Does An Atom Have Volume.
From www.broadlearnings.com
Atomic Structure Broad Learnings Does An Atom Have Volume Atoms are incredibly small, typically around 100 picometers in diameter. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. A convenient unit of length for measuring. The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. In volume the. Does An Atom Have Volume.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology Does An Atom Have Volume About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. Atoms are incredibly small, typically around 100 picometers in diameter. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy. In volume the nucleus takes up only 10 −14 metres. Does An Atom Have Volume.
From sciencenotes.org
Learn the Parts of an Atom Does An Atom Have Volume The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy. The nucleus is composed of. It is now known that an atom has a positively charged nucleus that makes up more. Does An Atom Have Volume.
From bgbasicscience.blogspot.com
Lets Get Inside An Atom!! The Science Station Does An Atom Have Volume Atoms are incredibly small, typically around 100 picometers in diameter. For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. It is now known that an atom has a positively charged nucleus that makes up more. Does An Atom Have Volume.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Does An Atom Have Volume The nucleus is composed of. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000. Does An Atom Have Volume.
From sites.google.com
Structure of the Atom Unit G Review Does An Atom Have Volume It is now known that an atom has a positively charged nucleus that makes up more than 99.9% of the atom’s mass but only about 1/100,000 of its volume. The nucleus is composed of. Atoms are incredibly small, typically around 100 picometers in diameter. The volume of an atom is about 15 orders of magnitude larger than the volume of. Does An Atom Have Volume.
From www.science-sparks.com
A Brief History of the Atom Does An Atom Have Volume The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. Atoms are incredibly small, typically around 100 picometers in diameter. In volume the nucleus takes up only 10 −14 metres of the space. Does An Atom Have Volume.
From www.biologyonline.com
Atom Definition and Examples Biology Online Dictionary Does An Atom Have Volume The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy. Atoms are incredibly small, typically around 100 picometers in diameter. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. In volume the nucleus takes up only 10 −14 metres. Does An Atom Have Volume.
From www.livescience.com
What Is an Atom? Live Science Does An Atom Have Volume The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy. The nucleus is composed of. For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus.. Does An Atom Have Volume.
From mumleyscience.weebly.com
The Atom Mumley Science Does An Atom Have Volume The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. The nucleus is composed of. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. It is now known that an atom has a positively charged nucleus that makes. Does An Atom Have Volume.
From www.slideserve.com
PPT Unit 2 Review Chemistry PowerPoint Presentation, free download Does An Atom Have Volume In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. It is now known that an atom has a positively charged nucleus that makes up more than 99.9% of the atom’s mass but only about 1/100,000 of its volume. The volume of an atom is about 15 orders of magnitude. Does An Atom Have Volume.
From classfullplymouth.z21.web.core.windows.net
Parts Of The Atom Does An Atom Have Volume In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. Atoms are incredibly small, typically around 100 picometers in diameter. It is now known that an atom has a positively charged. Does An Atom Have Volume.
From brainly.in
the volume of an atom is provided in some way by its...... Brainly.in Does An Atom Have Volume The nucleus is composed of. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy. For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. Atoms are incredibly small, typically around 100 picometers in diameter. It is now known that an atom has. Does An Atom Have Volume.
From chem.libretexts.org
2.3 The Modern View of Atomic Structure Chemistry LibreTexts Does An Atom Have Volume For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. A convenient unit of length for measuring. Atoms are incredibly small, typically around 100 picometers in diameter. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy. About 99.94% of an atom’s mass. Does An Atom Have Volume.
From profliway.hubpages.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Does An Atom Have Volume In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times. Does An Atom Have Volume.
From www.slideserve.com
PPT ATOMS & THE PERIODIC TABLE PowerPoint Presentation, free download Does An Atom Have Volume It is now known that an atom has a positively charged nucleus that makes up more than 99.9% of the atom’s mass but only about 1/100,000 of its volume. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. A convenient unit of length for measuring. About 99.94% of an. Does An Atom Have Volume.
From wghsjuniorscience.weebly.com
Atomic structure WGHS Junior Science Does An Atom Have Volume In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. Atoms are incredibly small, typically around 100 picometers in diameter. It is now known that an atom has a positively charged nucleus that makes. Does An Atom Have Volume.
From slideplayer.com
ATOMIC STRUCTURE CHAPTER ppt download Does An Atom Have Volume The nucleus is composed of. Atoms are incredibly small, typically around 100 picometers in diameter. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. The volume of. Does An Atom Have Volume.
From www.pathwaystochemistry.com
Atomic Structure Pathways to Chemistry Does An Atom Have Volume About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. A convenient unit of length for measuring. The nucleus is composed of. Atoms are incredibly small, typically around 100 picometers in diameter. It is now known that an atom has a positively charged nucleus that makes up more than 99.9% of. Does An Atom Have Volume.
From www.slideserve.com
PPT Subatomic Particles PowerPoint Presentation, free download ID Does An Atom Have Volume The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects. Does An Atom Have Volume.
From www.pathwaystochemistry.com
Atomic Structure Pathways to Chemistry Does An Atom Have Volume The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. The. Does An Atom Have Volume.
From www.worksheetsplanet.com
What is an Atom Meaning & Definition of Atom Does An Atom Have Volume For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. The nucleus is composed of. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier.. Does An Atom Have Volume.
From www.chem.fsu.edu
Electron Configurations Does An Atom Have Volume About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. The nucleus is composed of. For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. It is now known that an atom has a positively charged nucleus that makes up more than 99.9% of the. Does An Atom Have Volume.
From sciencing.com
How to Calculate the Volume of an Atom Sciencing Does An Atom Have Volume The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. A convenient unit of length for measuring. Atoms are incredibly small, typically around 100 picometers in diameter. The. Does An Atom Have Volume.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology Does An Atom Have Volume For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. Atoms are incredibly small, typically around 100 picometers in diameter. The nucleus contains the majority of an atom’s mass because protons and neutrons are much. Does An Atom Have Volume.
From sciencenotes.org
Periodic Table of Element Atom Sizes Does An Atom Have Volume In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. For uranium atom, the van der waals radius is about 186 pm = 1.86 ×10−10 m. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. The nucleus. Does An Atom Have Volume.
From www.slideserve.com
PPT The Parts of an Atom PowerPoint Presentation, free download ID Does An Atom Have Volume The volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. The volume of an object can generally be understood as the total measure of space that is unavailable for other objects to. About 99.94% of an atom’s mass is in its nucleus, with protons and neutrons being almost 2,000 times heavier. For. Does An Atom Have Volume.