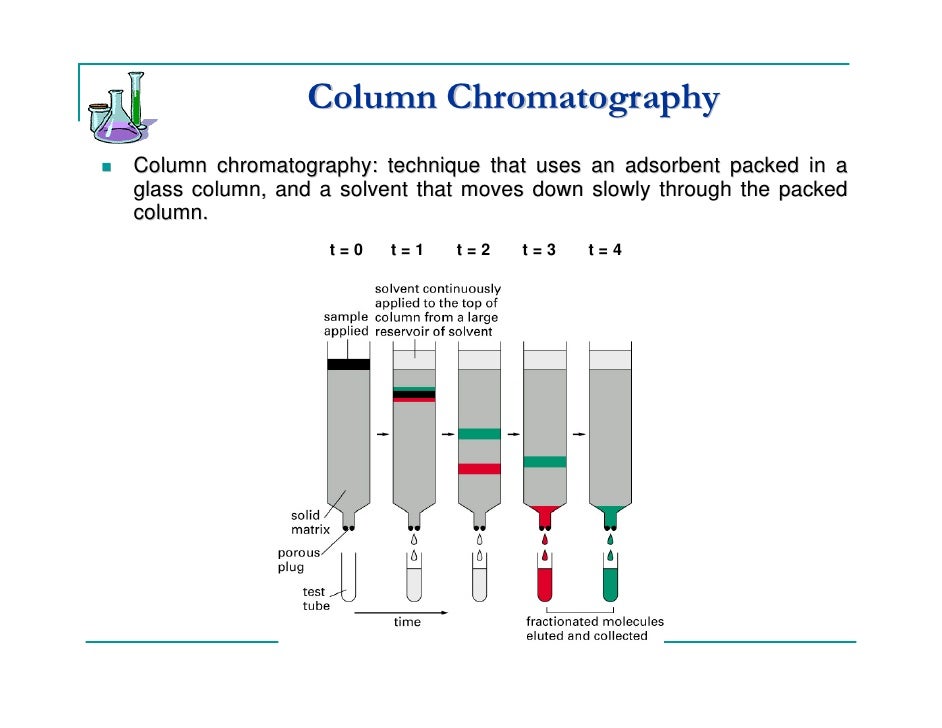
Column Chromatography Solvent Polarity . Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. Polar solvents can more effectively compete with the. Various sizes of chromatography columns are. When choosing a column, consider the polarity of both the stationary phase and your target analytes. If the stationary phase and analyte polarities are. This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column.
from www.slideshare.net
Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. If the stationary phase and analyte polarities are. Polar solvents can more effectively compete with the. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. Various sizes of chromatography columns are. Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. When choosing a column, consider the polarity of both the stationary phase and your target analytes.
Chromatography
Column Chromatography Solvent Polarity This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. If the stationary phase and analyte polarities are. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. When choosing a column, consider the polarity of both the stationary phase and your target analytes. Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. Polar solvents can more effectively compete with the. Various sizes of chromatography columns are.
From bitesizebio.com
Column Chromatography Made Simple An Easy to Follow Guide Column Chromatography Solvent Polarity Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. Various sizes of chromatography columns are. Of the two methods for bringing the stationary phase and the mobile phases into contact, the. Column Chromatography Solvent Polarity.
From www.slideserve.com
PPT Column Chromatography PowerPoint Presentation, free download ID Column Chromatography Solvent Polarity The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important. Column Chromatography Solvent Polarity.
From list.ly
Column Chromatography A Listly List Column Chromatography Solvent Polarity Polar solvents can more effectively compete with the. Various sizes of chromatography columns are. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. If the stationary phase and analyte polarities are. The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through. Column Chromatography Solvent Polarity.
From www.youtube.com
Selection of solvent in chromatography column chromatography Column Chromatography Solvent Polarity Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. Polar solvents can more effectively compete with the. Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. When choosing a column, consider the polarity of both the stationary phase and. Column Chromatography Solvent Polarity.
From www.slideshare.net
Chromatography Column Chromatography Solvent Polarity Various sizes of chromatography columns are. Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. Of the two methods for bringing the stationary phase and the mobile. Column Chromatography Solvent Polarity.
From www.numerade.com
SOLVED In column chromatography, the elution power of a solvent is Column Chromatography Solvent Polarity This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. Polar solvents can more effectively compete with the. Various sizes of chromatography columns are. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. The polarity of the solvent which. Column Chromatography Solvent Polarity.
From www.slideserve.com
PPT COLUMN CHROMATOGRAPHY PowerPoint Presentation, free download ID Column Chromatography Solvent Polarity Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. Various sizes of chromatography columns are. Flash column chromatography is usually carried out with a mixture of. Column Chromatography Solvent Polarity.
From mavink.com
Thin Layer Chromatography Polarity Column Chromatography Solvent Polarity Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. Various sizes of chromatography columns are. Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. Of the two methods for bringing the stationary phase and the mobile phases into contact, the. Column Chromatography Solvent Polarity.
From www.chegg.com
Solved Determine the solvent polarity index for the Column Chromatography Solvent Polarity When choosing a column, consider the polarity of both the stationary phase and your target analytes. Various sizes of chromatography columns are. Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. Column chromatography works on a much larger scale by packing the same materials into a vertical glass. Column Chromatography Solvent Polarity.
From www.goldbio.com
How Column Chromatography Works to Separate Proteins GoldBio Column Chromatography Solvent Polarity Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. If the stationary phase and analyte polarities are. Polar solvents can more effectively compete with the. Various sizes of chromatography columns are. Column chromatography works on a much larger scale by packing the same materials into a vertical glass. Column Chromatography Solvent Polarity.
From www.coursehero.com
[Solved] In regards to column chromatography, how does the polarity of Column Chromatography Solvent Polarity Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. Polar solvents can more effectively compete with the. If the stationary phase and analyte polarities are. This simply means that you. Column Chromatography Solvent Polarity.
From www.chemistrylearner.com
Column Chromatography Definition, Purpose, Types & Applications Column Chromatography Solvent Polarity Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column.. Column Chromatography Solvent Polarity.
From chemistnotes.com
Normal phase column chromatography Introduction, easy principle, uses Column Chromatography Solvent Polarity Polar solvents can more effectively compete with the. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. Various sizes of chromatography columns are. The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. When choosing a column, consider the. Column Chromatography Solvent Polarity.
From www.numerade.com
SOLVED Thin layer chromatography works on the same polarity principles Column Chromatography Solvent Polarity Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. If the stationary phase and analyte polarities are. Various sizes of chromatography columns are. This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. Polar solvents can more. Column Chromatography Solvent Polarity.
From klagyprqy.blob.core.windows.net
Thin Layer Chromatography Stationary Phase Polarity at Daniel Copeland blog Column Chromatography Solvent Polarity Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. If the stationary phase and analyte polarities are. This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. Flash column chromatography is usually carried out with a mixture of two. Column Chromatography Solvent Polarity.
From www.slideserve.com
PPT BASED ON POLARITY PowerPoint Presentation, free download ID1430840 Column Chromatography Solvent Polarity This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. When choosing a column, consider the polarity of both the stationary phase and your target analytes. If the stationary phase and analyte polarities are. Various sizes of chromatography columns are. Of the two methods for bringing the stationary. Column Chromatography Solvent Polarity.
From exyoddixr.blob.core.windows.net
How Does Polarity Affect Column Chromatography at Mattie Hudson blog Column Chromatography Solvent Polarity The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. If the stationary phase and analyte polarities are. This simply means that you increase the polarity of the solvent running. Column Chromatography Solvent Polarity.
From mavink.com
Solvent Polarity Chart For Tlc Column Chromatography Solvent Polarity When choosing a column, consider the polarity of both the stationary phase and your target analytes. Polar solvents can more effectively compete with the. Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. Column chromatography works on a much larger scale by packing the same materials into a vertical. Column Chromatography Solvent Polarity.
From mungfali.com
Solvent Polarity Chart Column Chromatography Solvent Polarity Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification.. Column Chromatography Solvent Polarity.
From mavink.com
Polarity Chart Of Solvents Column Chromatography Solvent Polarity The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. Various sizes of chromatography columns are. If the stationary phase and analyte polarities are. Of the two methods for bringing. Column Chromatography Solvent Polarity.
From exyoddixr.blob.core.windows.net
How Does Polarity Affect Column Chromatography at Mattie Hudson blog Column Chromatography Solvent Polarity If the stationary phase and analyte polarities are. When choosing a column, consider the polarity of both the stationary phase and your target analytes. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. The polarity of the solvent which is passed through the column affects the relative rates at which compounds. Column Chromatography Solvent Polarity.
From www.slideshare.net
Planar Chromatography Column Chromatography Solvent Polarity Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important. Column Chromatography Solvent Polarity.
From www.researchgate.net
Solvent polarity and eluotropic strength (ɛ°) Download Scientific Diagram Column Chromatography Solvent Polarity The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. If the stationary phase and analyte polarities are. Column chromatography works on a much larger scale by. Column Chromatography Solvent Polarity.
From chemistryhall.com
Thin Layer Chromatography A Complete Guide to TLC Column Chromatography Solvent Polarity If the stationary phase and analyte polarities are. The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. Various sizes of chromatography columns are. Column chromatography works on. Column Chromatography Solvent Polarity.
From alexmistry.z13.web.core.windows.net
Polarity Of Solvents In Chromatography Column Chromatography Solvent Polarity This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. Flash column chromatography is usually carried out with a mixture of two solvents, with a polar. Column Chromatography Solvent Polarity.
From pe.agrify.com
Column Chromatography The Definitive Guide Column Chromatography Solvent Polarity If the stationary phase and analyte polarities are. Polar solvents can more effectively compete with the. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. The polarity of the solvent which. Column Chromatography Solvent Polarity.
From www.numerade.com
SOLVED How does the polarity of the solvent affect TLC and Column Column Chromatography Solvent Polarity Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. Various sizes of chromatography columns are. When choosing a column, consider the polarity of both the stationary phase and your target analytes. If the stationary phase and analyte polarities are. Flash column chromatography is usually carried out with a. Column Chromatography Solvent Polarity.
From studylib.net
How to Perform a Column Chromatography Column Chromatography Solvent Polarity Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. Various sizes of chromatography columns are. If the stationary phase and analyte polarities are. When choosing a column, consider the polarity of both the stationary phase and your target analytes. Of the two methods for bringing the stationary phase and. Column Chromatography Solvent Polarity.
From www.slideserve.com
PPT Chromatography PowerPoint Presentation, free download ID2769389 Column Chromatography Solvent Polarity This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. If the stationary phase and analyte polarities are. Flash column chromatography is usually carried out with a mixture of two. Column Chromatography Solvent Polarity.
From mavink.com
Gc Column Polarity Chart Column Chromatography Solvent Polarity Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. Polar solvents can more effectively compete with the. The polarity of the solvent which is passed through the. Column Chromatography Solvent Polarity.
From www.researchgate.net
Nature of solvents, its polarity and general uses Download Scientific Column Chromatography Solvent Polarity Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. Polar solvents can more effectively compete with the. If the stationary phase and analyte polarities are. This simply means that you. Column Chromatography Solvent Polarity.
From bceweb.org
Solvent Polarity Chart A Visual Reference of Charts Chart Master Column Chromatography Solvent Polarity Various sizes of chromatography columns are. Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. If the stationary phase and analyte polarities are. Polar solvents can more effectively compete with the. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column.. Column Chromatography Solvent Polarity.
From joijfnvxn.blob.core.windows.net
Column Chromatography Class 12 at David Stokes blog Column Chromatography Solvent Polarity The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. When choosing a column, consider the polarity of both the stationary phase and your target analytes. Flash. Column Chromatography Solvent Polarity.
From studyingchemistry.tumblr.com
Life Of A Chemist Column chromatography Column Chromatography Solvent Polarity Polar solvents can more effectively compete with the. Of the two methods for bringing the stationary phase and the mobile phases into contact, the most important is column chromatography. The polarity of the solvent which is passed through the column affects the relative rates at which compounds move through the column. Column chromatography works on a much larger scale by. Column Chromatography Solvent Polarity.
From www.researchgate.net
Solvent ratio used for column chromatography Download Scientific Diagram Column Chromatography Solvent Polarity This simply means that you increase the polarity of the solvent running through the column (eluent) throughout the course of the purification. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. Various sizes of chromatography columns are. Flash column chromatography is usually carried out with a mixture of two solvents, with. Column Chromatography Solvent Polarity.