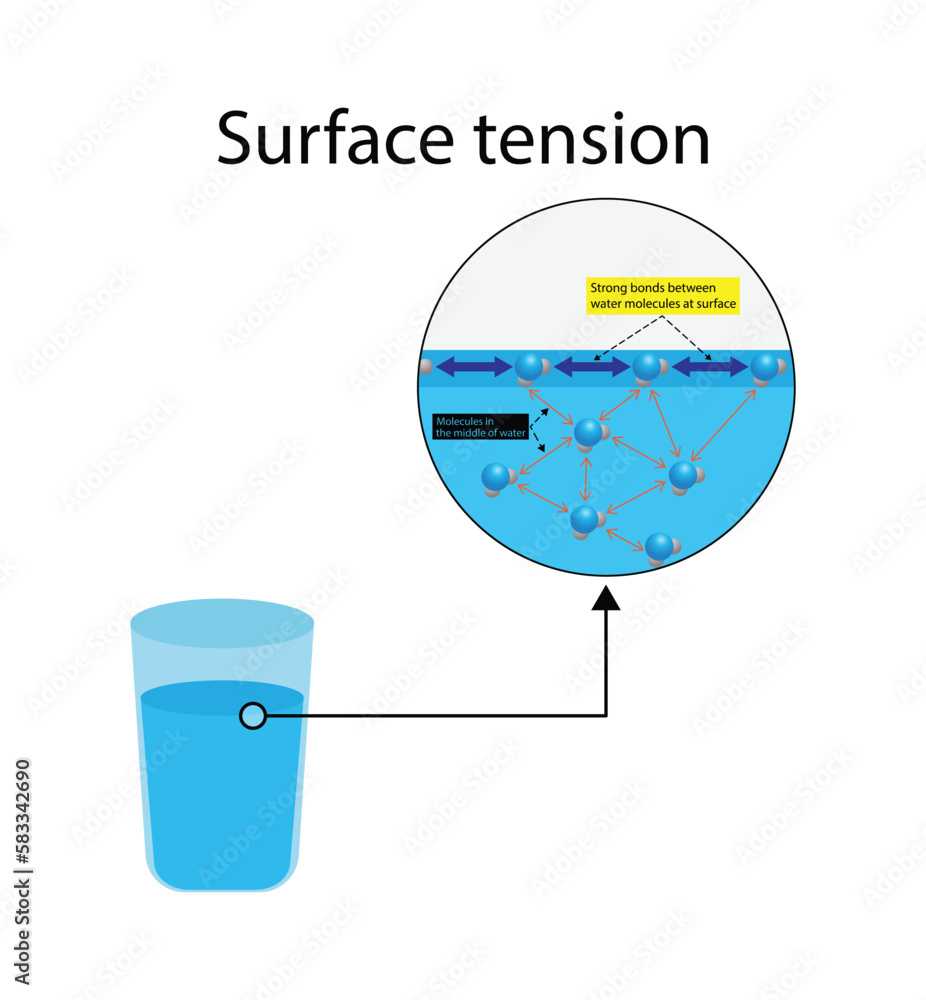
How Surface Tension Is Created . Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Thus, surface tension formula can be expressed as: Equivalently, it can be stated as surface energy in ergs per square centimeter. The polarity of water molecules can help explain why water has a strong surface tension. Surface tension is given by the ratio of the surface force (f) to the length of the force acting (l). Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension is caused by the effects of intermolecular forces at the interface. The molecules on the surface of a liquid are attracted by their neighbors from the. The attraction of molecules at the surface of a liquid is called surface tension.
from stock.adobe.com
Surface tension is caused by the effects of intermolecular forces at the interface. The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The polarity of water molecules can help explain why water has a strong surface tension. Thus, surface tension formula can be expressed as: Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension depends on the nature of the liquid, the surrounding environment and temperature.
illustration of physics, Surface tension of water, the cohesive forces
How Surface Tension Is Created Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The polarity of water molecules can help explain why water has a strong surface tension. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Thus, surface tension formula can be expressed as: Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is given by the ratio of the surface force (f) to the length of the force acting (l). The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension is caused by the effects of intermolecular forces at the interface. The attraction of molecules at the surface of a liquid is called surface tension. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How Surface Tension Is Created The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension, property of a liquid surface displayed by its acting as if it were a. How Surface Tension Is Created.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How Surface Tension Is Created Thus, surface tension formula can be expressed as: The molecules on the surface of a liquid are attracted by their neighbors from the. Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension, property of a liquid surface displayed by. How Surface Tension Is Created.
From www.vecteezy.com
Surface tension concept vector illustration 21669879 Vector Art at Vecteezy How Surface Tension Is Created Thus, surface tension formula can be expressed as: Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is caused by the effects of intermolecular forces at the interface. The polarity of water molecules can help explain why water has a strong surface tension. The attraction of molecules. How Surface Tension Is Created.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Surface Tension Is Created The polarity of water molecules can help explain why water has a strong surface tension. Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension depends on the nature of the liquid, the surrounding environment. How Surface Tension Is Created.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) How Surface Tension Is Created Since these intermolecular forces vary depending on the nature of the liquid (e.g. The attraction of molecules at the surface of a liquid is called surface tension. Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Thus, surface tension formula. How Surface Tension Is Created.
From almerja.com
Surface Tension How Surface Tension Is Created The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a phenomenon that occurs due to the cohesive forces of. How Surface Tension Is Created.
From www.slideserve.com
PPT Intermolecular Forces, Liquids and Solids PowerPoint Presentation How Surface Tension Is Created Surface tension is given by the ratio of the surface force (f) to the length of the force acting (l). Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Equivalently, it can be stated as surface energy in ergs per square centimeter. The molecules on the surface of a liquid are attracted by their. How Surface Tension Is Created.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector How Surface Tension Is Created Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Thus, surface tension formula can be expressed as: Equivalently, it can be stated. How Surface Tension Is Created.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson How Surface Tension Is Created The attraction of molecules at the surface of a liquid is called surface tension. The molecules on the surface of a liquid are attracted by their neighbors from the. The polarity of water molecules can help explain why water has a strong surface tension. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film. How Surface Tension Is Created.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID How Surface Tension Is Created The polarity of water molecules can help explain why water has a strong surface tension. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension depends on the nature of the liquid, the surrounding environment and temperature. The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is typically. How Surface Tension Is Created.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Surface Tension Is Created Surface tension is caused by the effects of intermolecular forces at the interface. The attraction of molecules at the surface of a liquid is called surface tension. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of. How Surface Tension Is Created.
From www.youtube.com
The Meaning of Surface Tension and its Practical Applications YouTube How Surface Tension Is Created Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The polarity of water molecules can help explain why water has a strong surface. How Surface Tension Is Created.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How Surface Tension Is Created The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The polarity of water molecules can help explain why water has a strong surface tension. Surface tension is the energy, or work, required to increase the surface area of a. How Surface Tension Is Created.
From www.pinterest.com
Surface Tension Force Properties of Water Surface tension, Elementary How Surface Tension Is Created Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The polarity of water molecules can help explain why water has a strong surface tension. The molecules on the surface of a liquid are attracted by. How Surface Tension Is Created.
From www.bqua.com
What is Surface Tension Surface Tension Definition BQUA How Surface Tension Is Created Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Surface tension is the energy, or work, required to increase the. How Surface Tension Is Created.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Surface Tension Is Created Surface tension is given by the ratio of the surface force (f) to the length of the force acting (l). Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Surface tension is caused by the effects of intermolecular forces at the interface.. How Surface Tension Is Created.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and How Surface Tension Is Created Thus, surface tension formula can be expressed as: Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension depends on the nature of the liquid, the surrounding environment and. How Surface Tension Is Created.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How Surface Tension Is Created Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is caused by the effects of intermolecular forces at the interface.. How Surface Tension Is Created.
From www.youtube.com
Surface Tension of Water Explained YouTube How Surface Tension Is Created Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is given by the ratio of the surface force (f) to the length of the force acting (l). Surface tension depends on the. How Surface Tension Is Created.
From recemev.jimdo.com
Surface Tension recemev How Surface Tension Is Created Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Thus, surface tension formula can be expressed as: The polarity of water molecules can help explain why water has a strong surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due. How Surface Tension Is Created.
From www.worksheetsplanet.com
What is Surface Tension? How Surface Tension Is Created The attraction of molecules at the surface of a liquid is called surface tension. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension is given by the ratio of the surface force (f) to the length of the force acting (l). The polarity of water molecules can help explain why water has a strong. How Surface Tension Is Created.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 How Surface Tension Is Created Thus, surface tension formula can be expressed as: Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Surface tension is given by the ratio of the surface force (f) to the length of the force acting (l). The molecules on. How Surface Tension Is Created.
From www.youtube.com
Surface Tension YouTube How Surface Tension Is Created Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. The polarity of water molecules can help explain why water has a strong surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary. How Surface Tension Is Created.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Surface Tension Is Created The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Thus, surface tension formula can be expressed as: Surface tension. How Surface Tension Is Created.
From www.sliderbase.com
Properties of Liquids Presentation Chemistry How Surface Tension Is Created Equivalently, it can be stated as surface energy in ergs per square centimeter. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Thus, surface tension formula can be expressed as: Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Surface tension is typically measured in dynes/cm, the force in dynes required. How Surface Tension Is Created.
From www.adda247.com
Surface Tension Definition, Formula, Examples How Surface Tension Is Created The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension depends on the nature of the liquid, the surrounding environment and temperature. The polarity of water molecules can help explain why water has a strong surface tension. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film. How Surface Tension Is Created.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID6036532 How Surface Tension Is Created Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Since these intermolecular forces vary. How Surface Tension Is Created.
From blog.biolinscientific.com
What is surface tension? How Surface Tension Is Created Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Since these intermolecular forces vary depending. How Surface Tension Is Created.
From courses.lumenlearning.com
Surface Tension Introduction to Chemistry How Surface Tension Is Created Thus, surface tension formula can be expressed as: Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface of a liquid are attracted by their neighbors from the. The polarity of water molecules can help explain why water has a strong surface tension. Surface tension. How Surface Tension Is Created.
From www.learnatnoon.com
What is Surface Tension in Physics? How Surface Tension Is Created The attraction of molecules at the surface of a liquid is called surface tension. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Surface tension is typically. How Surface Tension Is Created.
From premeditatedleftovers.com
Surface Tension Science Experiment for Kids How Surface Tension Is Created Thus, surface tension formula can be expressed as: Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is a phenomenon that. How Surface Tension Is Created.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Surface Tension Is Created The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is caused by. How Surface Tension Is Created.
From byjus.com
Explain the surface tension phenomenon with examples. How Surface Tension Is Created The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is given by the ratio of the surface force (f) to the length of the force acting (l). Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a phenomenon. How Surface Tension Is Created.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How Surface Tension Is Created The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. The attraction of molecules at the surface of a liquid is called surface tension. Equivalently, it can be stated as surface energy in ergs per. How Surface Tension Is Created.
From www.youtube.com
How does Surface Tension work? YouTube How Surface Tension Is Created Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The polarity of water molecules can help explain why water has a strong surface tension. Thus, surface tension formula can be expressed as: The attraction of molecules at the surface of a liquid is called surface tension. Since these intermolecular forces vary depending on the. How Surface Tension Is Created.