Heat Of Reaction From Heat Of Combustion . The heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. Calculating heat of reaction from heat of formation. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. An application of hess's law allows us to use standard heats of formation to. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. See the molar heat of combustion or hhv for common fuels and examples of problems. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants.
from lessonstone.z13.web.core.windows.net
The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Calculating heat of reaction from heat of formation. See the molar heat of combustion or hhv for common fuels and examples of problems. An application of hess's law allows us to use standard heats of formation to. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. The heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel.
Formula Of Heat Of Formation
Heat Of Reaction From Heat Of Combustion Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. An application of hess's law allows us to use standard heats of formation to. See the molar heat of combustion or hhv for common fuels and examples of problems. Calculating heat of reaction from heat of formation. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. The heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel.
From www.wikihow.com
How to Calculate Heat of Combustion 12 Steps (with Pictures) Heat Of Reaction From Heat Of Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. An application of hess's law allows us to use standard heats of. Heat Of Reaction From Heat Of Combustion.
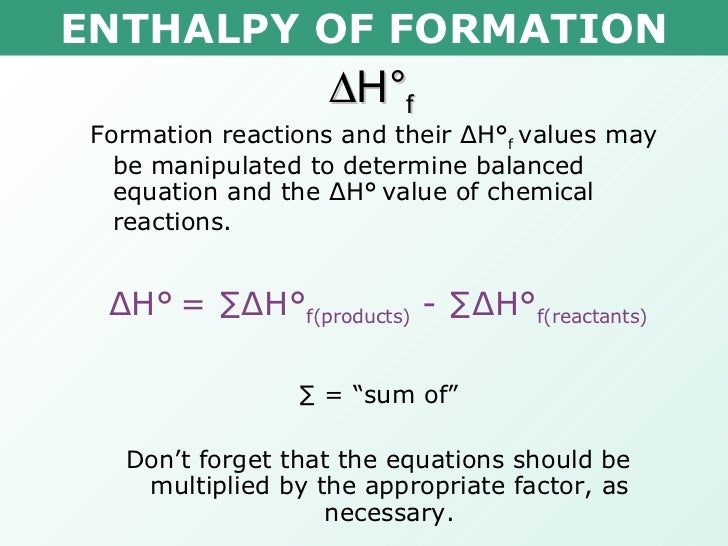
From www.slideserve.com
PPT Energetics PowerPoint Presentation ID1204656 Heat Of Reaction From Heat Of Combustion Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. The heat of formation or enthalpy of formation is the standard heat of reaction for forming. Heat Of Reaction From Heat Of Combustion.
From www.youtube.com
Heat of Reaction (from Heat of Formation) YouTube Heat Of Reaction From Heat Of Combustion See the molar heat of combustion or hhv for common fuels and examples of problems. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. An application of hess's law allows us to use standard heats of formation to. The heat of formation or enthalpy. Heat Of Reaction From Heat Of Combustion.
From printablekopierdu.z21.web.core.windows.net
Heat Of Reaction Formula Heat Of Reaction From Heat Of Combustion The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. Learn how to calculate the heat of combustion (energy. Heat Of Reaction From Heat Of Combustion.
From spmchemistry.blog.onlinetuition.com.my
Heat of Reaction SPM Chemistry Heat Of Reaction From Heat Of Combustion See the molar heat of combustion or hhv for common fuels and examples of problems. An application of hess's law allows us to use standard heats of formation to. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Learn how to calculate the. Heat Of Reaction From Heat Of Combustion.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Heat Of Reaction From Heat Of Combustion Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. Calculating heat of reaction from heat of formation. Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. See the molar heat of combustion. Heat Of Reaction From Heat Of Combustion.
From www.slideserve.com
PPT Advanced Thermodynamics Note 3 Heat Effects PowerPoint Heat Of Reaction From Heat Of Combustion The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Calculating heat of reaction from heat of formation. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. The. Heat Of Reaction From Heat Of Combustion.
From printableaachen.z19.web.core.windows.net
How To Calculate The Heat Of Reaction Heat Of Reaction From Heat Of Combustion Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. Calculating heat of reaction from heat of formation. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. The heat of combustion is a. Heat Of Reaction From Heat Of Combustion.
From www.slideserve.com
PPT CLASSIFYING CHEMICAL REACTIONS PowerPoint Presentation ID1330610 Heat Of Reaction From Heat Of Combustion An application of hess's law allows us to use standard heats of formation to. The heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. Calculating heat of reaction from heat of formation. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product,. Heat Of Reaction From Heat Of Combustion.
From pediaa.com
Difference Between Heat of Formation and Heat of Reaction Definition Heat Of Reaction From Heat Of Combustion An application of hess's law allows us to use standard heats of formation to. See the molar heat of combustion or hhv for common fuels and examples of problems. Calculating heat of reaction from heat of formation. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products. Heat Of Reaction From Heat Of Combustion.
From www.slideserve.com
PPT Heat of Reaction PowerPoint Presentation, free download ID6434217 Heat Of Reaction From Heat Of Combustion Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. To calculate the heat of combustion, use hess’s law,. Heat Of Reaction From Heat Of Combustion.
From spmchemistry.blog.onlinetuition.com.my
Heat of Combustion SPM Chemistry Heat Of Reaction From Heat Of Combustion See the molar heat of combustion or hhv for common fuels and examples of problems. Calculating heat of reaction from heat of formation. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. An application of hess's law allows us to use standard heats of. Heat Of Reaction From Heat Of Combustion.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Heat Of Reaction From Heat Of Combustion See the molar heat of combustion or hhv for common fuels and examples of problems. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Calculating heat of reaction from heat of formation. Learn how to calculate the heat of combustion (energy content) of. Heat Of Reaction From Heat Of Combustion.
From www.youtube.com
Heat of reactions and heat of combustion (ALevels and IB chemistry Heat Of Reaction From Heat Of Combustion The heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. Calculating heat of reaction from heat of formation. The heat of formation or enthalpy of formation is the standard. Heat Of Reaction From Heat Of Combustion.
From www.chegg.com
Solved 3. Calculate the heat of reaction at 25 °C for the Heat Of Reaction From Heat Of Combustion Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. An application of hess's law allows us to use standard heats of formation to. See the molar heat of combustion or hhv for common fuels and examples of problems. The heat of combustion is a useful calculation for analyzing. Heat Of Reaction From Heat Of Combustion.
From www.numerade.com
SOLVED a. Use standard heats of formation to determine the heat of Heat Of Reaction From Heat Of Combustion The heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. Calculating heat of reaction from heat of formation. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. The heat of formation or enthalpy of formation is. Heat Of Reaction From Heat Of Combustion.
From sciencenotes.org
Combustion Reaction Definition and Examples Heat Of Reaction From Heat Of Combustion Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. An application of hess's law allows us to use standard heats of formation to. Calculating heat of reaction from heat of formation. To calculate the heat of combustion, use hess’s law, which states that the. Heat Of Reaction From Heat Of Combustion.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Heat Of Reaction From Heat Of Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. See the molar heat of combustion or hhv for common fuels and examples of problems. Calculating heat of reaction from heat of formation. The heat of combustion is a useful calculation for analyzing the amount of energy. Heat Of Reaction From Heat Of Combustion.
From svanfitz.blogspot.com
Enthalpy Change Of Combustion PPT ENTHALPY OF FORMATION Combustion Heat Of Reaction From Heat Of Combustion Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. See the molar heat of combustion or hhv for common fuels and examples of. Heat Of Reaction From Heat Of Combustion.
From lessonschoolimbrowning.z14.web.core.windows.net
How To Determine Heat Of Reaction Heat Of Reaction From Heat Of Combustion See the molar heat of combustion or hhv for common fuels and examples of problems. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. An application of hess's law allows us to use standard heats of formation to. Learn how to calculate the heat of combustion. Heat Of Reaction From Heat Of Combustion.
From chemistrytalk.org
Heat of Reaction ChemTalk Heat Of Reaction From Heat Of Combustion The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. See the molar heat of combustion or hhv for common fuels and examples of problems. The heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. Learn. Heat Of Reaction From Heat Of Combustion.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1794758 Heat Of Reaction From Heat Of Combustion Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. An application of hess's law allows us to use standard heats of formation to. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting. Heat Of Reaction From Heat Of Combustion.
From worksheetmagicrusso.z21.web.core.windows.net
How To Determine The Heat Of Formation Heat Of Reaction From Heat Of Combustion Calculating heat of reaction from heat of formation. See the molar heat of combustion or hhv for common fuels and examples of problems. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Learn how to calculate the heat of combustion of a substance. Heat Of Reaction From Heat Of Combustion.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Heat Of Reaction From Heat Of Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. Calculating heat of reaction from heat of formation. Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. The heat of combustion is a useful calculation. Heat Of Reaction From Heat Of Combustion.
From www.youtube.com
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Heat Of Reaction From Heat Of Combustion Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. An application of hess's law allows us to use standard heats of formation to.. Heat Of Reaction From Heat Of Combustion.
From sites.google.com
Unit 2B Chemical Reactions and Thermochemistry UIA (Department of Heat Of Reaction From Heat Of Combustion The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. The heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. Calculating heat of reaction from heat of formation. An application of hess's law allows us to. Heat Of Reaction From Heat Of Combustion.
From dokumen.tips
(PPT) ENERGY AND REACTIONS ENDOTHERMIC AND EXOTHERMIC REACTIONS HEAT OF Heat Of Reaction From Heat Of Combustion Calculating heat of reaction from heat of formation. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. An. Heat Of Reaction From Heat Of Combustion.
From www.slideserve.com
PPT Chapter 17 “Thermochemistry” PowerPoint Presentation, free Heat Of Reaction From Heat Of Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. Calculating heat of reaction from heat of formation. See the molar heat. Heat Of Reaction From Heat Of Combustion.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation ID1832797 Heat Of Reaction From Heat Of Combustion Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. Calculating heat of reaction from heat of formation. See the molar heat of combustion or hhv for common fuels and examples of problems. The heat of formation or enthalpy of formation is the standard heat. Heat Of Reaction From Heat Of Combustion.
From www.slideserve.com
PPT Heat of Combustion PowerPoint Presentation, free download ID Heat Of Reaction From Heat Of Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. An application of hess's law allows us to use standard heats of formation to. Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. The heat. Heat Of Reaction From Heat Of Combustion.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID2429720 Heat Of Reaction From Heat Of Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. Learn how to calculate the heat of combustion (energy content) of a. Heat Of Reaction From Heat Of Combustion.
From quizzlistreplevies.z13.web.core.windows.net
How To Calculate The Heat Of Formation Heat Of Reaction From Heat Of Combustion Learn how to calculate the heat of combustion of a substance by using a bomb calorimeter and the enthalpy of formation of products and reactants. Calculating heat of reaction from heat of formation. An application of hess's law allows us to use standard heats of formation to. The heat of combustion is a useful calculation for analyzing the amount of. Heat Of Reaction From Heat Of Combustion.
From www.slideserve.com
PPT Chapter 11 Thermochemistry Heat and Chemical Change PowerPoint Heat Of Reaction From Heat Of Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. Calculating heat of reaction from heat of formation. The heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. See the molar heat of combustion or hhv for common fuels. Heat Of Reaction From Heat Of Combustion.
From www.nagwa.com
Question Video Calculating the Standard Heat of Reaction for the Heat Of Reaction From Heat Of Combustion An application of hess's law allows us to use standard heats of formation to. Learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation and the. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. Learn how. Heat Of Reaction From Heat Of Combustion.
From lessonstone.z13.web.core.windows.net
Formula Of Heat Of Formation Heat Of Reaction From Heat Of Combustion The heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. Calculating heat of reaction from heat of formation. An application of hess's law allows us to use standard heats of formation to. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product,. Heat Of Reaction From Heat Of Combustion.