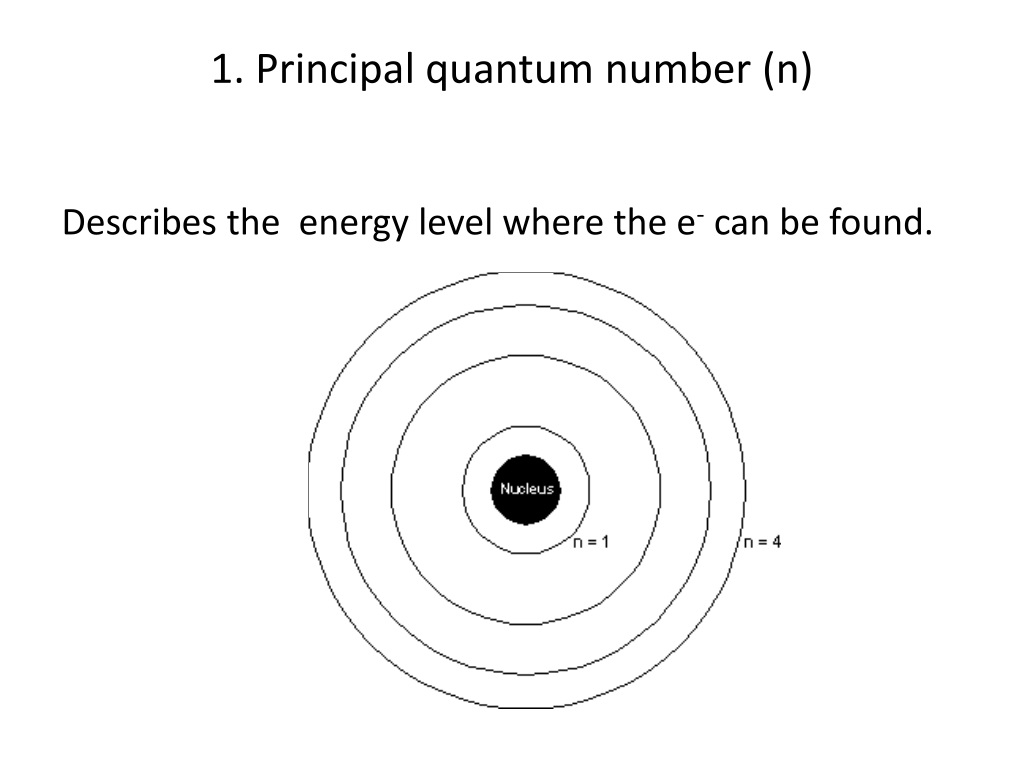
Energy Magnetic Quantum Number . Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. Every electron localized in an atom can be described by four quantum numbers. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. The angular momentum quantum number determines the. The potential energy levels are described by the main quantum number n and by the secondary quantum number l. In atoms, there are a total of four quantum numbers: Let us study the third quantum number in the set. The principal quantum number defines the general value of the electronic energy. It is used to project the angular momentum. The probability distributions are given by the secondary quantum. The magnetic quantum number of an electron is one of the four quantum numbers that state the position of the electron with respect to the. The pauli exclusion principle tells us that no two. What is magnetic quantum number. The magnetic quantum number divides the subshell into orbitals and determines their number.
from www.slideserve.com
The potential energy levels are described by the main quantum number n and by the secondary quantum number l. The pauli exclusion principle tells us that no two. It is used to project the angular momentum. In atoms, there are a total of four quantum numbers: What is magnetic quantum number. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. The magnetic quantum number of an electron is one of the four quantum numbers that state the position of the electron with respect to the. Every electron localized in an atom can be described by four quantum numbers. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. Let us study the third quantum number in the set.
PPT Quantum Numbers and Electronic Configuration PowerPoint
Energy Magnetic Quantum Number The angular momentum quantum number determines the. The probability distributions are given by the secondary quantum. Let us study the third quantum number in the set. It is used to project the angular momentum. The magnetic quantum number of an electron is one of the four quantum numbers that state the position of the electron with respect to the. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. What is magnetic quantum number. The pauli exclusion principle tells us that no two. The principal quantum number defines the general value of the electronic energy. Every electron localized in an atom can be described by four quantum numbers. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. In atoms, there are a total of four quantum numbers: The potential energy levels are described by the main quantum number n and by the secondary quantum number l. The angular momentum quantum number determines the. The magnetic quantum number divides the subshell into orbitals and determines their number.
From www.youtube.com
Orbitals, Quantum Numbers & Electron Configuration Multiple Choice Energy Magnetic Quantum Number The principal quantum number defines the general value of the electronic energy. Let us study the third quantum number in the set. It is used to project the angular momentum. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. The probability distributions are given by the secondary. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Arrangement of Electrons in Atoms PowerPoint Presentation, free Energy Magnetic Quantum Number The potential energy levels are described by the main quantum number n and by the secondary quantum number l. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. The pauli exclusion principle tells us that no two. It is used to project the angular momentum. Every electron. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT AP Chemistry Unit 3 Elements PowerPoint Presentation, free Energy Magnetic Quantum Number In atoms, there are a total of four quantum numbers: The angular momentum quantum number determines the. The potential energy levels are described by the main quantum number n and by the secondary quantum number l. The principal quantum number defines the general value of the electronic energy. The principal quantum number (n), the orbital angular momentum quantum number (l),. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 Energy Magnetic Quantum Number The magnetic quantum number of an electron is one of the four quantum numbers that state the position of the electron with respect to the. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. The magnetic quantum number divides the subshell into orbitals and determines their number.. Energy Magnetic Quantum Number.
From savekhaoyai.blogspot.com
41 quantum number practice worksheet Worksheet Database Energy Magnetic Quantum Number The pauli exclusion principle tells us that no two. The probability distributions are given by the secondary quantum. The magnetic quantum number of an electron is one of the four quantum numbers that state the position of the electron with respect to the. The angular momentum quantum number determines the. Let us study the third quantum number in the set.. Energy Magnetic Quantum Number.
From www.brainkart.com
Quantum numbers Energy Magnetic Quantum Number What is magnetic quantum number. The probability distributions are given by the secondary quantum. The potential energy levels are described by the main quantum number n and by the secondary quantum number l. Let us study the third quantum number in the set. The magnetic quantum number divides the subshell into orbitals and determines their number. The magnetic quantum number. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 Energy Magnetic Quantum Number It is used to project the angular momentum. What is magnetic quantum number. The potential energy levels are described by the main quantum number n and by the secondary quantum number l. Let us study the third quantum number in the set. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Electronic Structure of Atoms PowerPoint Presentation, free Energy Magnetic Quantum Number In atoms, there are a total of four quantum numbers: It is used to project the angular momentum. The probability distributions are given by the secondary quantum. The principal quantum number defines the general value of the electronic energy. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more. Energy Magnetic Quantum Number.
From slidetodoc.com
Quantum Numbers THE FOUR QUANTUM NUMBERS Quantum Numbers Energy Magnetic Quantum Number The pauli exclusion principle tells us that no two. The potential energy levels are described by the main quantum number n and by the secondary quantum number l. The magnetic quantum number of an electron is one of the four quantum numbers that state the position of the electron with respect to the. In atoms, there are a total of. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT QUANTUM MECHANICAL MODEL OF THE ATOM PowerPoint Presentation Energy Magnetic Quantum Number Let us study the third quantum number in the set. What is magnetic quantum number. The probability distributions are given by the secondary quantum. The potential energy levels are described by the main quantum number n and by the secondary quantum number l. The magnetic quantum number divides the subshell into orbitals and determines their number. The angular momentum quantum. Energy Magnetic Quantum Number.
From in.pinterest.com
quantum number. Chemistry lessons, Teaching chemistry, Quantum Energy Magnetic Quantum Number Let us study the third quantum number in the set. The pauli exclusion principle tells us that no two. The angular momentum quantum number determines the. The magnetic quantum number divides the subshell into orbitals and determines their number. What is magnetic quantum number. In atoms, there are a total of four quantum numbers: The potential energy levels are described. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers and Electronic Configuration PowerPoint Energy Magnetic Quantum Number Let us study the third quantum number in the set. The probability distributions are given by the secondary quantum. The magnetic quantum number divides the subshell into orbitals and determines their number. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. The angular momentum quantum number determines. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation ID2135857 Energy Magnetic Quantum Number The pauli exclusion principle tells us that no two. The angular momentum quantum number determines the. The potential energy levels are described by the main quantum number n and by the secondary quantum number l. The magnetic quantum number divides the subshell into orbitals and determines their number. The principal quantum number (n), the orbital angular momentum quantum number (l),. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Unit 2 PowerPoint Presentation, free download ID298336 Energy Magnetic Quantum Number The magnetic quantum number divides the subshell into orbitals and determines their number. It is used to project the angular momentum. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. The principal quantum number defines the general value of the electronic energy. In atoms, there are a. Energy Magnetic Quantum Number.
From testbook.com
Quantum Number Learn Definition, Formula, Applications Energy Magnetic Quantum Number The principal quantum number defines the general value of the electronic energy. The magnetic quantum number divides the subshell into orbitals and determines their number. It is used to project the angular momentum. The angular momentum quantum number determines the. In atoms, there are a total of four quantum numbers: The potential energy levels are described by the main quantum. Energy Magnetic Quantum Number.
From www.sliderbase.com
Electron Quantum Numbers Presentation Chemistry Energy Magnetic Quantum Number Let us study the third quantum number in the set. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. It is used to project the angular momentum. The pauli exclusion principle tells us that no two. The principal quantum number defines the general value of the electronic. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Atomic Structure and Periodicity PowerPoint Presentation, free Energy Magnetic Quantum Number The potential energy levels are described by the main quantum number n and by the secondary quantum number l. The pauli exclusion principle tells us that no two. What is magnetic quantum number. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. Every electron localized in an atom can. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum numbers and orbital energies Each atom’s electron has a Energy Magnetic Quantum Number What is magnetic quantum number. In atoms, there are a total of four quantum numbers: Let us study the third quantum number in the set. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. The principal quantum number (n), the orbital angular momentum quantum number (l), the. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Mechanics PowerPoint Presentation, free download ID1084898 Energy Magnetic Quantum Number What is magnetic quantum number. The magnetic quantum number divides the subshell into orbitals and determines their number. The angular momentum quantum number determines the. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s. Energy Magnetic Quantum Number.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps Energy Magnetic Quantum Number The magnetic quantum number divides the subshell into orbitals and determines their number. Let us study the third quantum number in the set. It is used to project the angular momentum. The angular momentum quantum number determines the. The magnetic quantum number of an electron is one of the four quantum numbers that state the position of the electron with. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Arrangement of Electrons in Atoms PowerPoint Presentation, free Energy Magnetic Quantum Number It is used to project the angular momentum. The angular momentum quantum number determines the. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. The principal quantum number defines the general value of the electronic energy. What is magnetic quantum number. The magnetic quantum number divides the subshell into. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Chemistry I Honors Unit 3 Quantum Mechanical Model of the Atom Energy Magnetic Quantum Number Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. The potential energy levels are described by the main quantum number n and by the secondary quantum number l. The magnetic quantum number divides the subshell into orbitals and determines their number. The probability distributions are given by. Energy Magnetic Quantum Number.
From api-project-1022638073839.appspot.com
What is a set of four quantum numbers that could represent the last Energy Magnetic Quantum Number The principal quantum number defines the general value of the electronic energy. What is magnetic quantum number. The potential energy levels are described by the main quantum number n and by the secondary quantum number l. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. Magnetic quantum number (m. Energy Magnetic Quantum Number.
From shanimchemistry.blogspot.com
*CHEMISTRY MATRICULATION* QUANTUM NUMBERS Energy Magnetic Quantum Number It is used to project the angular momentum. The angular momentum quantum number determines the. The pauli exclusion principle tells us that no two. The potential energy levels are described by the main quantum number n and by the secondary quantum number l. What is magnetic quantum number. The magnetic quantum number divides the subshell into orbitals and determines their. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID4952601 Energy Magnetic Quantum Number Every electron localized in an atom can be described by four quantum numbers. The probability distributions are given by the secondary quantum. The pauli exclusion principle tells us that no two. It is used to project the angular momentum. The principal quantum number defines the general value of the electronic energy. Magnetic quantum number (m l) the magnetic quantum number. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Chapter 28 PowerPoint Presentation, free download ID1104026 Energy Magnetic Quantum Number What is magnetic quantum number. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. The probability distributions are given by the secondary quantum. In atoms, there are a total of four quantum numbers: The magnetic quantum number of an electron is one of the four quantum numbers. Energy Magnetic Quantum Number.
From www.chemistrylearner.com
Physical Chemistry Page 2 of 3 Chemistry Learner Energy Magnetic Quantum Number The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. Every electron localized in an atom can be described by four quantum numbers. Let us study the third quantum number in the set. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel. Energy Magnetic Quantum Number.
From chemistnotes.com
Quantum Numbers, Types Principal, Azimuthal, and Spin Energy Magnetic Quantum Number In atoms, there are a total of four quantum numbers: The potential energy levels are described by the main quantum number n and by the secondary quantum number l. Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. It is used to project the angular momentum. The. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Principle Quantum Numbers PowerPoint Presentation, free download Energy Magnetic Quantum Number What is magnetic quantum number. The potential energy levels are described by the main quantum number n and by the secondary quantum number l. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. The magnetic quantum number divides the subshell into orbitals and determines their number. The probability distributions. Energy Magnetic Quantum Number.
From study.com
Quantum Numbers on the Periodic Table Definition & Overview Lesson Energy Magnetic Quantum Number The magnetic quantum number of an electron is one of the four quantum numbers that state the position of the electron with respect to the. It is used to project the angular momentum. Let us study the third quantum number in the set. Every electron localized in an atom can be described by four quantum numbers. The potential energy levels. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Mechanics PowerPoint Presentation, free download ID9428728 Energy Magnetic Quantum Number The angular momentum quantum number determines the. The magnetic quantum number of an electron is one of the four quantum numbers that state the position of the electron with respect to the. The pauli exclusion principle tells us that no two. What is magnetic quantum number. Magnetic quantum number (m l) the magnetic quantum number describes the split in the. Energy Magnetic Quantum Number.
From www.numerade.com
quantum number specifies Energy Magnetic Quantum Number The pauli exclusion principle tells us that no two. The principal quantum number defines the general value of the electronic energy. In atoms, there are a total of four quantum numbers: The magnetic quantum number divides the subshell into orbitals and determines their number. The potential energy levels are described by the main quantum number n and by the secondary. Energy Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers Activity PowerPoint Presentation, free download Energy Magnetic Quantum Number Magnetic quantum number (m l) the magnetic quantum number describes the split in the electron’s energy sublevel into two or more levels. In atoms, there are a total of four quantum numbers: Let us study the third quantum number in the set. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and. Energy Magnetic Quantum Number.
From byjus.com
Quantum Number Definition Schrodinger Equation Energy Magnetic Quantum Number What is magnetic quantum number. The magnetic quantum number of an electron is one of the four quantum numbers that state the position of the electron with respect to the. The pauli exclusion principle tells us that no two. Every electron localized in an atom can be described by four quantum numbers. In atoms, there are a total of four. Energy Magnetic Quantum Number.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps Energy Magnetic Quantum Number Every electron localized in an atom can be described by four quantum numbers. The magnetic quantum number divides the subshell into orbitals and determines their number. The angular momentum quantum number determines the. Let us study the third quantum number in the set. What is magnetic quantum number. In atoms, there are a total of four quantum numbers: The principal. Energy Magnetic Quantum Number.