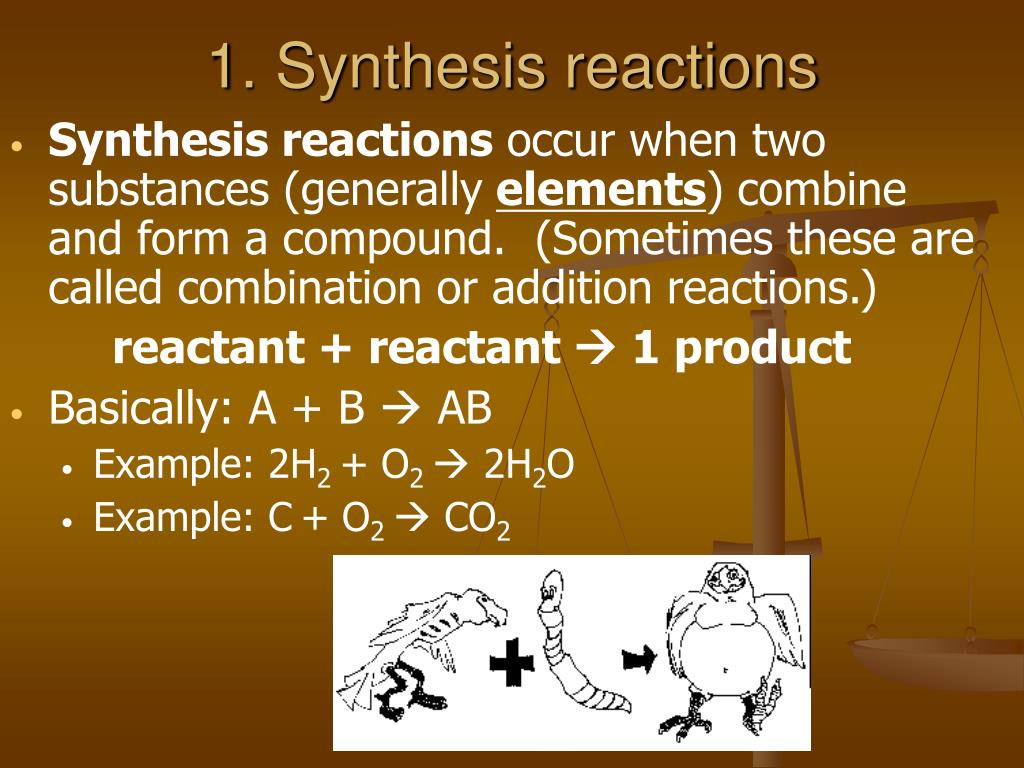
Synthesis Reaction Definition Chemistry . Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. A synthesis reaction is when two or more chemicals combine and form a more complex product. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. Synthesis reactions are exothermic reactions. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A + b → ab. That means that two pieces join together to. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product.
from www.slideserve.com
A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction is when two or more chemicals combine and form a more complex product. A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. That means that two pieces join together to. Synthesis reactions are exothermic reactions. A + b → ab.
PPT Chemical Reactions PowerPoint Presentation, free download ID
Synthesis Reaction Definition Chemistry Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. Synthesis reactions are exothermic reactions. A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product. A synthesis reaction is when two or more chemicals combine and form a more complex product. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A + b → ab. That means that two pieces join together to.
From slideplayer.com
Chemical Reactions Unit ppt download Synthesis Reaction Definition Chemistry A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. That means that two pieces. Synthesis Reaction Definition Chemistry.
From www.pinterest.co.uk
Synthesis Chemistry Chemistry, Synthesis, Chemical structure Synthesis Reaction Definition Chemistry Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A + b → ab. Synthesis reactions are exothermic reactions. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product. A. Synthesis Reaction Definition Chemistry.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Synthesis Reaction Definition Chemistry A synthesis reaction is when two or more chemicals combine and form a more complex product. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. That means that two pieces join together to. A synthesis. Synthesis Reaction Definition Chemistry.
From www.thoughtco.com
Synthesis Reaction Description Plus Examples Synthesis Reaction Definition Chemistry Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A synthesis. Synthesis Reaction Definition Chemistry.
From www.vrogue.co
Chemical Reaction Definition Equations Examples Types vrogue.co Synthesis Reaction Definition Chemistry A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. Synthesis reactions are exothermic reactions. A synthesis reaction or direct combination reaction is one of the most common types. Synthesis Reaction Definition Chemistry.
From slidetodoc.com
Lesson 32 What is a synthesis reaction Chemical Synthesis Reaction Definition Chemistry A synthesis reaction is when two or more chemicals combine and form a more complex product. That means that two pieces join together to. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis reactions are exothermic reactions. Synthesis are, at this. Synthesis Reaction Definition Chemistry.
From slideplayer.com
Types of Chemical Reactions ppt download Synthesis Reaction Definition Chemistry A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A + b → ab. A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product. That means that two pieces join together. Synthesis Reaction Definition Chemistry.
From sciencenotes.org
What Is a Synthesis Reaction? Definition and Examples Synthesis Reaction Definition Chemistry That means that two pieces join together to. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product.. Synthesis Reaction Definition Chemistry.
From www.thoughtco.com
Types of Chemical Reactions (With Examples) Synthesis Reaction Definition Chemistry A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction is when two or more chemicals combine and form a more complex product. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product.. Synthesis Reaction Definition Chemistry.
From slideplayer.com
Types of Chemical Reactions ppt download Synthesis Reaction Definition Chemistry That means that two pieces join together to. Synthesis reactions are exothermic reactions. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product. Synthesis are, at this introductory level, almost always the. Synthesis Reaction Definition Chemistry.
From slideplayer.com
Chemical Equations & Reactions ppt download Synthesis Reaction Definition Chemistry A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction. Synthesis Reaction Definition Chemistry.
From slideplayer.com
The Last Chapter on Chemistry ppt download Synthesis Reaction Definition Chemistry That means that two pieces join together to. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. Synthesis reactions are exothermic reactions. A synthesis reaction or direct combination reaction is one of the most common types of chemical. Synthesis Reaction Definition Chemistry.
From www.vrogue.co
Synthesis Combination Reaction Definition Examples An vrogue.co Synthesis Reaction Definition Chemistry Synthesis reactions are exothermic reactions. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. Learn the general form, types and examples of synthesis reactions in chemistry, including organic. Synthesis Reaction Definition Chemistry.
From ar.inspiredpencil.com
Synthesis Reaction Examples Synthesis Reaction Definition Chemistry Synthesis reactions are exothermic reactions. A + b → ab. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction is when two or more chemicals combine and form a more complex product. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. Synthesis are, at. Synthesis Reaction Definition Chemistry.
From www.worksheetsplanet.com
Synthesis Reaction Characteristics Synthesis Reaction Definition Chemistry Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. That means that two pieces join together to. A synthesis reaction is when two or more chemicals combine and form a more complex product. Synthesis reactions are exothermic reactions.. Synthesis Reaction Definition Chemistry.
From www.vrogue.co
Synthesis Chemical Reaction A Synthesis Reaction Or D vrogue.co Synthesis Reaction Definition Chemistry A + b → ab. Synthesis reactions are exothermic reactions. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. A synthesis reaction is when two or more chemicals combine and form a more complex product. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction or direct combination. Synthesis Reaction Definition Chemistry.
From www.vrogue.co
Synthesis Reaction Definition And Examples Chemistry vrogue.co Synthesis Reaction Definition Chemistry Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. A synthesis reaction is when two or more chemicals combine and form a more complex product. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Synthesis are, at this introductory level, almost always the reverse. Synthesis Reaction Definition Chemistry.
From www.vrogue.co
Synthesis Combination Reaction Definition Examples An vrogue.co Synthesis Reaction Definition Chemistry A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A + b → ab. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. That means that two pieces join together to. Synthesis reactions are. Synthesis Reaction Definition Chemistry.
From www.slideserve.com
PPT Vocabulary PowerPoint Presentation, free download ID1428098 Synthesis Reaction Definition Chemistry A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A + b → ab. Synthesis reactions are exothermic reactions. Synthesis are, at this introductory level,. Synthesis Reaction Definition Chemistry.
From slideplayer.com
Quiz 5.1 In a chemical equation, “(aq)” means “aqueous”. What does this Synthesis Reaction Definition Chemistry A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Synthesis reactions are exothermic reactions. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A + b → ab. A synthesis reaction is defined as. Synthesis Reaction Definition Chemistry.
From soniafersgibson.blogspot.com
Which of the Following Describes a Synthesis Reaction Synthesis Reaction Definition Chemistry A synthesis reaction is when two or more chemicals combine and form a more complex product. Synthesis reactions are exothermic reactions. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. That means that two pieces join together to. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction.. Synthesis Reaction Definition Chemistry.
From www.vrogue.co
Synthesis Combination Reaction Definition Examples An vrogue.co Synthesis Reaction Definition Chemistry A + b → ab. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product. A synthesis reaction. Synthesis Reaction Definition Chemistry.
From www.slideserve.com
PPT Synthesis Reaction PowerPoint Presentation, free download ID Synthesis Reaction Definition Chemistry A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction is when two or more. Synthesis Reaction Definition Chemistry.
From www.dreamstime.com
Synthesis Reaction Infographic Diagram Stock Vector Illustration of Synthesis Reaction Definition Chemistry Synthesis reactions are exothermic reactions. A + b → ab. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. That means that two pieces join together to. A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product. Synthesis reactions are reactions. Synthesis Reaction Definition Chemistry.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Synthesis Reaction Definition Chemistry A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. That means. Synthesis Reaction Definition Chemistry.
From slideplayer.com
Types of Reactions. ppt download Synthesis Reaction Definition Chemistry A + b → ab. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. Synthesis reactions are exothermic reactions. A synthesis reaction is when two or more chemicals combine and form a more complex product. A synthesis reaction. Synthesis Reaction Definition Chemistry.
From slideplayer.com
Chapter 6 Chemical Reactions ppt download Synthesis Reaction Definition Chemistry A synthesis reaction is when two or more chemicals combine and form a more complex product. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction.. Synthesis Reaction Definition Chemistry.
From slideplayer.com
If the rate of a reaction is going to ppt download Synthesis Reaction Definition Chemistry A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction is when two or more chemicals combine and form a more complex product. Learn the general form, types and examples of synthesis reactions in chemistry, including. Synthesis Reaction Definition Chemistry.
From www.pinterest.com.au
What Is a Synthesis Reaction? Synthesis Reaction Definition Chemistry That means that two pieces join together to. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product. A synthesis reaction or direct combination reaction is a type of chemical reaction in. Synthesis Reaction Definition Chemistry.
From slideplayer.com
The Evil Twin to ppt download Synthesis Reaction Definition Chemistry Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis reactions. Synthesis Reaction Definition Chemistry.
From www.vrogue.co
Synthesis Combination Reaction Definition Examples An vrogue.co Synthesis Reaction Definition Chemistry A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis reactions are exothermic reactions. A + b → ab. That means that two pieces join together to. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. Synthesis. Synthesis Reaction Definition Chemistry.
From www.slideserve.com
PPT The 5 Types of Reactions PowerPoint Presentation, free download Synthesis Reaction Definition Chemistry A synthesis reaction is when two or more chemicals combine and form a more complex product. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. That means that two pieces join together to. A synthesis reaction or direct. Synthesis Reaction Definition Chemistry.
From slideplayer.com
Learning Objectives To check our understanding of balanced equations Synthesis Reaction Definition Chemistry That means that two pieces join together to. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Learn the general form, types and examples of synthesis reactions in chemistry, including organic and inorganic compounds. A synthesis reaction is when two or more chemicals combine and form a more complex product. A synthesis. Synthesis Reaction Definition Chemistry.
From peopleareawesomepics.blogspot.com
peopleareawesomepics Synthesis Reaction Definition Chemistry A synthesis reaction is defined as a process in chemistry that involves identifying an optimal reaction path to produce a product. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction is when two or more chemicals combine and form a more complex product. Synthesis reactions are reactions that involve. Synthesis Reaction Definition Chemistry.
From www.vrogue.co
What Is A Synthesis Reaction Definition And Examples vrogue.co Synthesis Reaction Definition Chemistry Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. A synthesis reaction is when two or more chemicals combine and form a more complex product. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction or direct combination reaction is one of the most common types of chemical. Synthesis Reaction Definition Chemistry.