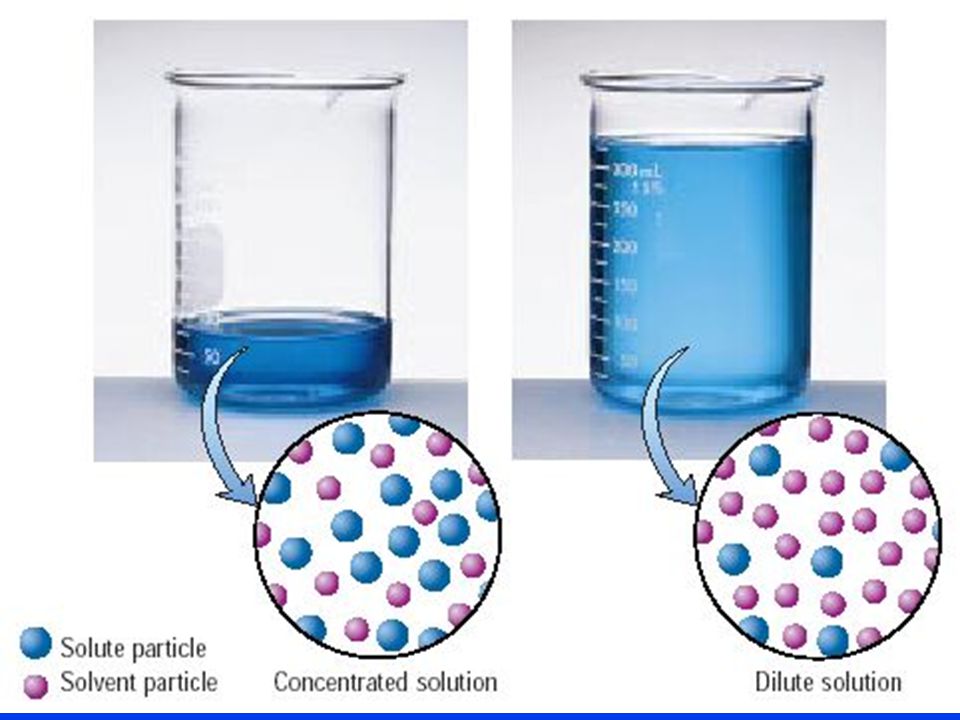
Chemistry Water Dilution Problems . A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. How many ml of this solution. Problem \(\pageindex{2}\) what does it mean when we say that a. Of course, the resulting solution is. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. these dilution example problems show how to perform the calculations needed to make a diluted. the concentration and the volumes change in a dilution. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. I add 560 ml more water to it? 2) if i dilute 250 ml of 0.10 m. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. dilutions of stock (or standard) solutions. to dilute a solution means to add more solvent without the addition of more solute. Imagine we have a salt water solution with a certain concentration.
from socratic.org
Imagine we have a salt water solution with a certain concentration. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. How many ml of this solution. A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. Problem \(\pageindex{2}\) what does it mean when we say that a. 2) if i dilute 250 ml of 0.10 m. these dilution example problems show how to perform the calculations needed to make a diluted. to dilute a solution means to add more solvent without the addition of more solute. I add 560 ml more water to it? dilutions of stock (or standard) solutions.
How can I calculate the dilution factor using concentration? Socratic
Chemistry Water Dilution Problems 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. the concentration and the volumes change in a dilution. Imagine we have a salt water solution with a certain concentration. 2) if i dilute 250 ml of 0.10 m. I add 560 ml more water to it? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. these dilution example problems show how to perform the calculations needed to make a diluted. dilutions of stock (or standard) solutions. Problem \(\pageindex{2}\) what does it mean when we say that a. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. to dilute a solution means to add more solvent without the addition of more solute. How many ml of this solution. Of course, the resulting solution is.
From printablezoneunglad.z13.web.core.windows.net
How To Calculate Dilution Concentrations Chemistry Water Dilution Problems this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. I add 560 ml more water to it? dilutions of stock (or standard) solutions. to dilute a solution means to add more solvent without the addition of more solute. How many ml of this solution. If you dilute 175 ml of. Chemistry Water Dilution Problems.
From www.pinterest.com
DILUTIONS M1V1; M2V2 Solving Dilution Problems in Solution Chemistry Chemistry Water Dilution Problems 2) if i dilute 250 ml of 0.10 m. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Problem \(\pageindex{2}\) what does it mean when we say that a. to dilute a solution means to add more solvent without the addition of more solute. dilutions. Chemistry Water Dilution Problems.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic Chemistry Water Dilution Problems the concentration and the volumes change in a dilution. A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. Imagine we have a salt water solution with a certain concentration. Problem \(\pageindex{2}\) what does it mean when we say that a. dilutions of stock (or standard) solutions. this chemistry. Chemistry Water Dilution Problems.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube Chemistry Water Dilution Problems 2) if i dilute 250 ml of 0.10 m. How many ml of this solution. the concentration and the volumes change in a dilution. Imagine we have a salt water solution with a certain concentration. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. If you dilute 175 ml of. Chemistry Water Dilution Problems.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Chemistry Water Dilution Problems Of course, the resulting solution is. A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. I add 560 ml more water to it? Problem \(\pageindex{2}\) what does it mean when we say that a. these dilution example problems show how to perform the calculations needed to make a diluted. . Chemistry Water Dilution Problems.
From dxojiglxm.blob.core.windows.net
Serial Dilution Sample Problems With Answers at Michael Forbes blog Chemistry Water Dilution Problems A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. these dilution example problems show how to perform the calculations needed to make a diluted. How many ml of this solution. I add 560 ml more water to it? 2) if i dilute 250 ml of 0.10 m. 1) if i. Chemistry Water Dilution Problems.
From www.youtube.com
How to do Dilution problems? Solved examples YouTube Chemistry Water Dilution Problems Imagine we have a salt water solution with a certain concentration. Of course, the resulting solution is. to dilute a solution means to add more solvent without the addition of more solute. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. these dilution example problems show how to perform the. Chemistry Water Dilution Problems.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Chemistry Water Dilution Problems How many ml of this solution. these dilution example problems show how to perform the calculations needed to make a diluted. to dilute a solution means to add more solvent without the addition of more solute. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution.. Chemistry Water Dilution Problems.
From www.youtube.com
Molarity How to solve dilution problems Chemtutorfree YouTube Chemistry Water Dilution Problems 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Problem \(\pageindex{2}\) what does it mean when we say that a. How many ml of this solution. Of course, the resulting solution is. A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. . Chemistry Water Dilution Problems.
From giozdcywk.blob.core.windows.net
Chemical Dispenser Backflow Preventer at Geraldine Cook blog Chemistry Water Dilution Problems Problem \(\pageindex{2}\) what does it mean when we say that a. I add 560 ml more water to it? A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. to dilute a solution means to add more solvent without the addition of more solute. this chemistry video tutorial explains how. Chemistry Water Dilution Problems.
From pdfprof.com
concentration of solutions practice problems Chemistry Water Dilution Problems Of course, the resulting solution is. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. How many ml of this solution. 2) if i dilute 250 ml of 0.10 m. Imagine we have a salt water solution with a certain concentration. I add 560 ml more water to it? these. Chemistry Water Dilution Problems.
From giodasqsk.blob.core.windows.net
Dilutions And Molarity Worksheet at Michael Farley blog Chemistry Water Dilution Problems this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. the concentration and the volumes change in a dilution. to dilute a solution means to add more solvent without the addition of more solute. How many ml of this solution. these dilution example problems show how to perform the calculations. Chemistry Water Dilution Problems.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Chemistry Water Dilution Problems A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. dilutions of stock (or standard) solutions. Problem \(\pageindex{2}\) what does it mean when we say that a. to dilute a solution means to add more solvent without the addition of more solute. If you dilute 175 ml of a 1.6. Chemistry Water Dilution Problems.
From www.youtube.com
Dilutions Explained with Problems YouTube Chemistry Water Dilution Problems 2) if i dilute 250 ml of 0.10 m. dilutions of stock (or standard) solutions. to dilute a solution means to add more solvent without the addition of more solute. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. the concentration and the volumes change in a dilution. Problem. Chemistry Water Dilution Problems.
From giodasqsk.blob.core.windows.net
Dilutions And Molarity Worksheet at Michael Farley blog Chemistry Water Dilution Problems 2) if i dilute 250 ml of 0.10 m. Of course, the resulting solution is. I add 560 ml more water to it? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. to dilute a solution means to add more solvent without the addition of more solute. Problem \(\pageindex{2}\) what. Chemistry Water Dilution Problems.
From www.youtube.com
Dilution Method Problems Waste Water Engineering YouTube Chemistry Water Dilution Problems 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 2) if i dilute 250 ml of 0.10 m. Imagine we have a salt water solution with a certain concentration. How many ml of this solution. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine. Chemistry Water Dilution Problems.
From pressurewashingresource.com
Chem dilution chart 2 by Chemical/Chemistry Chemistry Water Dilution Problems the concentration and the volumes change in a dilution. to dilute a solution means to add more solvent without the addition of more solute. A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine. Chemistry Water Dilution Problems.
From exoxgkatn.blob.core.windows.net
Dilution Problems With Answers at Michael Keller blog Chemistry Water Dilution Problems If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Problem \(\pageindex{2}\) what does it mean when we say that a. Imagine we have a salt water solution with a certain concentration. to dilute a solution means to add more solvent without the addition of more solute.. Chemistry Water Dilution Problems.
From candjwater.com
6 Benefits of Reverse Osmosis to Purify Your Tap Water c and j water Chemistry Water Dilution Problems these dilution example problems show how to perform the calculations needed to make a diluted. I add 560 ml more water to it? dilutions of stock (or standard) solutions. Problem \(\pageindex{2}\) what does it mean when we say that a. the concentration and the volumes change in a dilution. to dilute a solution means to add. Chemistry Water Dilution Problems.
From studyadvertiser.z21.web.core.windows.net
Calculate Final Concentration After Dilution Chemistry Water Dilution Problems 2) if i dilute 250 ml of 0.10 m. How many ml of this solution. the concentration and the volumes change in a dilution. these dilution example problems show how to perform the calculations needed to make a diluted. I add 560 ml more water to it? 1) if i have 340 ml of a 0.5 m nabr. Chemistry Water Dilution Problems.
From dxosnjafu.blob.core.windows.net
Dilution Calculation Videos at Iva Fugate blog Chemistry Water Dilution Problems A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. Of course, the resulting solution is. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. dilutions of stock (or standard) solutions. these dilution example problems show how to perform the calculations. Chemistry Water Dilution Problems.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Chemistry Water Dilution Problems Problem \(\pageindex{2}\) what does it mean when we say that a. 2) if i dilute 250 ml of 0.10 m. A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution.. Chemistry Water Dilution Problems.
From www.chemistryworksheet.com
Dilution Problems Chemistry Worksheet With Answers Chemistry Water Dilution Problems the concentration and the volumes change in a dilution. How many ml of this solution. Imagine we have a salt water solution with a certain concentration. dilutions of stock (or standard) solutions. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. If you dilute 175 ml of a 1.6. Chemistry Water Dilution Problems.
From studyschoolnucleator.z21.web.core.windows.net
Molarity By Dilution Worksheet Chemistry Water Dilution Problems 2) if i dilute 250 ml of 0.10 m. to dilute a solution means to add more solvent without the addition of more solute. How many ml of this solution. these dilution example problems show how to perform the calculations needed to make a diluted. If you dilute 175 ml of a 1.6 m solution of licl to. Chemistry Water Dilution Problems.
From www.youtube.com
Dilution Calculation Practice YouTube Chemistry Water Dilution Problems to dilute a solution means to add more solvent without the addition of more solute. I add 560 ml more water to it? the concentration and the volumes change in a dilution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. How many ml of this solution. dilutions. Chemistry Water Dilution Problems.
From mmerevise.co.uk
Concentrations and Dilutions MME Chemistry Water Dilution Problems these dilution example problems show how to perform the calculations needed to make a diluted. 2) if i dilute 250 ml of 0.10 m. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. the concentration and the volumes change in a dilution. I add 560 ml more water to it?. Chemistry Water Dilution Problems.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Chemistry Water Dilution Problems Problem \(\pageindex{2}\) what does it mean when we say that a. to dilute a solution means to add more solvent without the addition of more solute. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. these dilution example problems show how to perform the calculations needed to make a diluted.. Chemistry Water Dilution Problems.
From exosxhrzi.blob.core.windows.net
How To Dilute 2 Mg/Ml To 1 Mg/Ml at Freddie Forrester blog Chemistry Water Dilution Problems If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. dilutions of stock (or standard) solutions. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Problem \(\pageindex{2}\) what does it mean when we say that a. I add. Chemistry Water Dilution Problems.
From www.youtube.com
How To Dilute Chemicals Dilution Ratios Explained! YouTube Chemistry Water Dilution Problems Of course, the resulting solution is. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. dilutions of stock (or standard) solutions. I add 560 ml more water to it? How many ml of this solution. 1) if i have 340 ml of a 0.5 m nabr. Chemistry Water Dilution Problems.
From quizgorblimeys.z21.web.core.windows.net
What Is A Concentrated Solution Chemistry Water Dilution Problems Problem \(\pageindex{2}\) what does it mean when we say that a. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. to dilute a solution means to add more solvent without the addition of more solute. dilutions of stock (or standard) solutions. If you dilute 175 ml of a 1.6 m. Chemistry Water Dilution Problems.
From printablelibswathed.z19.web.core.windows.net
Molarity And Dilution Worksheet Chemistry Water Dilution Problems dilutions of stock (or standard) solutions. How many ml of this solution. I add 560 ml more water to it? Of course, the resulting solution is. to dilute a solution means to add more solvent without the addition of more solute. Problem \(\pageindex{2}\) what does it mean when we say that a. A 74.31 g sample of ba(oh). Chemistry Water Dilution Problems.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Chemistry Water Dilution Problems dilutions of stock (or standard) solutions. Problem \(\pageindex{2}\) what does it mean when we say that a. these dilution example problems show how to perform the calculations needed to make a diluted. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. the concentration and. Chemistry Water Dilution Problems.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Chemistry Water Dilution Problems dilutions of stock (or standard) solutions. A 74.31 g sample of ba(oh) 2 is dissolved in enough water to make 2.450 l of solution. the concentration and the volumes change in a dilution. Of course, the resulting solution is. Problem \(\pageindex{2}\) what does it mean when we say that a. 1) if i have 340 ml of a. Chemistry Water Dilution Problems.
From exodwyspp.blob.core.windows.net
Dilution Water Calculator at Mary Guevara blog Chemistry Water Dilution Problems these dilution example problems show how to perform the calculations needed to make a diluted. Imagine we have a salt water solution with a certain concentration. the concentration and the volumes change in a dilution. I add 560 ml more water to it? 1) if i have 340 ml of a 0.5 m nabr solution, what will the. Chemistry Water Dilution Problems.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Chemistry Water Dilution Problems dilutions of stock (or standard) solutions. to dilute a solution means to add more solvent without the addition of more solute. Imagine we have a salt water solution with a certain concentration. Of course, the resulting solution is. I add 560 ml more water to it? this chemistry video tutorial explains how to solve common dilution problems. Chemistry Water Dilution Problems.