Soap Water Chemical Formula . Its fundamental chemistry involves the combination of fats or oils with. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both. Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Soap is a classic cleaning agent that has been used for centuries. One the above structure, circle the portion of the molecule that. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. The reaction produces sodium salts of.
from www.alamy.com
Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both. Soap is a classic cleaning agent that has been used for centuries. Its fundamental chemistry involves the combination of fats or oils with. One the above structure, circle the portion of the molecule that. Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. The reaction produces sodium salts of.
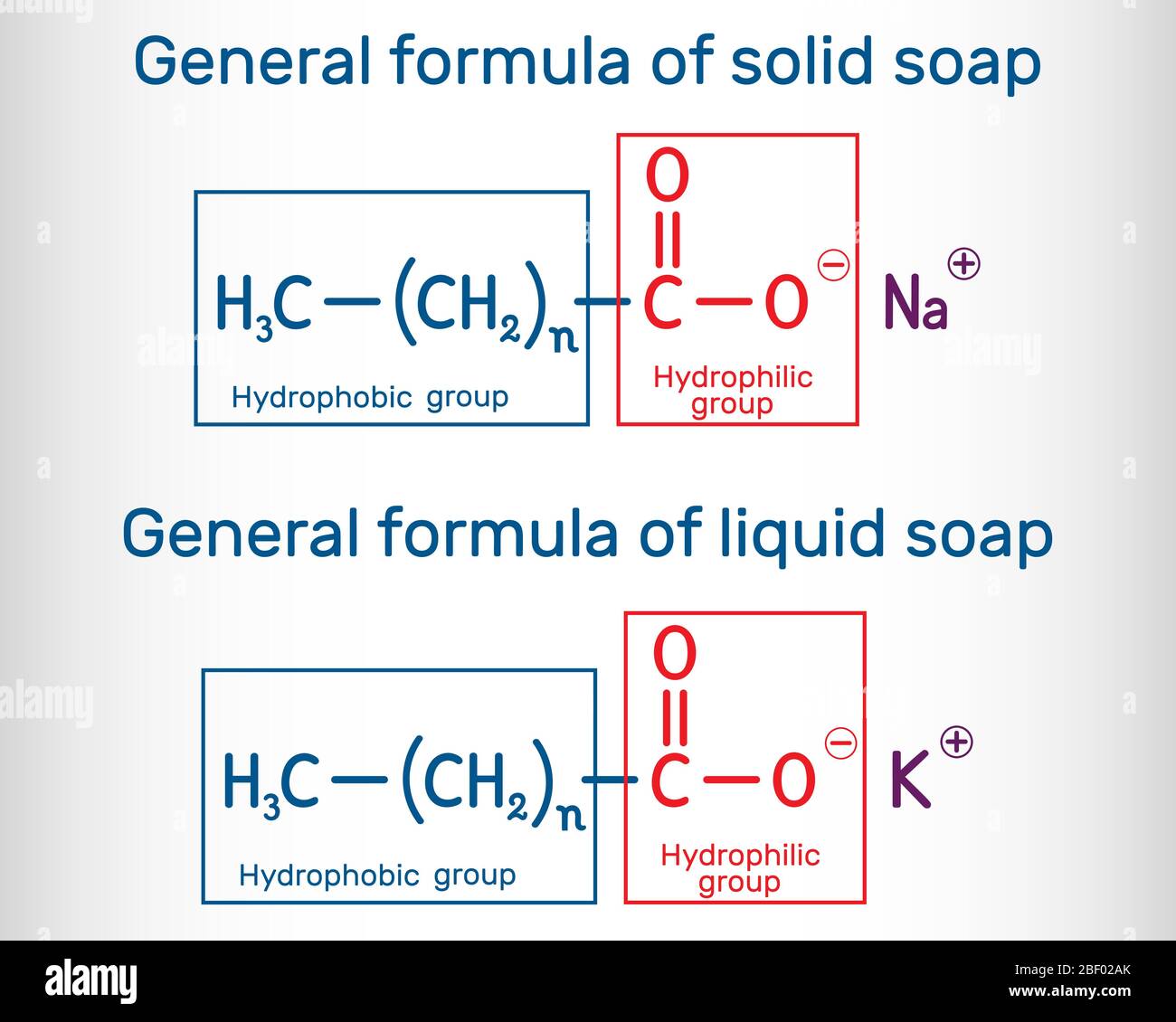
General formula of solid and liquid soap molecule. RCOONa, RCOOK
Soap Water Chemical Formula Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. The reaction produces sodium salts of. Soap is a classic cleaning agent that has been used for centuries. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both. Its fundamental chemistry involves the combination of fats or oils with. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. One the above structure, circle the portion of the molecule that.
From www.alamy.com
General formula of solid and liquid soap molecule. RCOONa, RCOOK Soap Water Chemical Formula Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Soap is a classic cleaning agent that has been used for centuries. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit. Soap Water Chemical Formula.
From gioenugqh.blob.core.windows.net
Soap Liquid Chemical Formula at Helen Weintraub blog Soap Water Chemical Formula Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Its fundamental chemistry involves. Soap Water Chemical Formula.
From www.slideshare.net
Soap and detergent ( chemistry folio form 5 ) Soap Water Chemical Formula The reaction produces sodium salts of. Its fundamental chemistry involves the combination of fats or oils with. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. One the above structure, circle the portion. Soap Water Chemical Formula.
From spmchemistry.blog.onlinetuition.com.my
Detergent SPM Chemistry Soap Water Chemical Formula Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Soap is a classic cleaning agent that has been used for centuries. The reaction produces sodium salts of. One the above structure, circle the portion of the molecule that. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due. Soap Water Chemical Formula.
From reportd953.web.fc2.com
What's the chemical formula of soap? Soap Water Chemical Formula Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both. One the above structure, circle the portion of the molecule that. Its fundamental chemistry involves the combination of fats or oils with. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Currently,. Soap Water Chemical Formula.
From ar.inspiredpencil.com
Soap Molecule Structure Soap Water Chemical Formula Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Its fundamental chemistry involves the combination of fats or oils with. Soap is a classic cleaning agent that has been used for centuries. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. The reaction. Soap Water Chemical Formula.
From www.tessshebaylo.com
Balanced Equation For Sodium Polyacrylate And Water Tessshebaylo Soap Water Chemical Formula Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soap is a classic cleaning agent that has been used for centuries. The reaction produces sodium salts of. Its fundamental chemistry involves the combination of fats or oils with. Carboxylic acids and salts having alkyl chains longer than eight. Soap Water Chemical Formula.
From thesoapmoleculeco.com
The Soap Molecule Co. Soap Water Chemical Formula Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. One the above structure, circle the portion of the molecule that. The reaction produces sodium salts of. Its fundamental chemistry involves the combination of fats or oils with. Currently, sodium carbonate or sodium hydroxide is used to neutralize the. Soap Water Chemical Formula.
From learning.sciencemuseumgroup.org.uk
Bubble Fun! Science Museum Group Learning Soap Water Chemical Formula Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Soap is a classic cleaning agent that has been used. Soap Water Chemical Formula.
From ar.inspiredpencil.com
Soap Molecule Structure Soap Water Chemical Formula Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. The reaction produces sodium salts of. One the above structure, circle the portion of the molecule that. Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior. Soap Water Chemical Formula.
From gioenugqh.blob.core.windows.net
Soap Liquid Chemical Formula at Helen Weintraub blog Soap Water Chemical Formula Soap is a classic cleaning agent that has been used for centuries. Its fundamental chemistry involves the combination of fats or oils with. The reaction produces sodium salts of. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. One the above structure, circle the portion of the molecule. Soap Water Chemical Formula.
From www.researchgate.net
1. Composition of bar of soap and liquid soap Download Table Soap Water Chemical Formula Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Its fundamental chemistry involves the combination of fats or oils with. The reaction produces sodium salts of. Soap is a classic cleaning agent that has been used for centuries. Revise the. Soap Water Chemical Formula.
From www.shutterstock.com
Saponification Equation Reaction Soap Chemistry Equation Stock Soap Water Chemical Formula Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Its fundamental chemistry involves the combination of fats or oils with. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soap is produced by a saponification or. Soap Water Chemical Formula.
From stock.adobe.com
Saponification equation, reaction of soap, chemistry equation of soap Soap Water Chemical Formula Its fundamental chemistry involves the combination of fats or oils with. Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both. Soap is produced by a saponification or basic hydrolysis reaction of a fat. Soap Water Chemical Formula.
From cosmosmagazine.com
The chemistry of soap Soap Water Chemical Formula Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Its fundamental chemistry involves the combination of fats or oils with. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual. Soap Water Chemical Formula.
From www.dreamstime.com
Set Chemical Formula for H2O, Heart with Water Drop, Washing Hands Soap Soap Water Chemical Formula Its fundamental chemistry involves the combination of fats or oils with. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both. Currently, sodium carbonate or sodium hydroxide. Soap Water Chemical Formula.
From ar.inspiredpencil.com
Preparation Of Soap Chemistry Soap Water Chemical Formula Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. The reaction produces sodium salts of. Soap is a classic cleaning agent that has been used for. Soap Water Chemical Formula.
From rightmultiprogram.weebly.com
Detergent Soap Making Formula Pdf rightmultiprogram Soap Water Chemical Formula The reaction produces sodium salts of. Its fundamental chemistry involves the combination of fats or oils with. One the above structure, circle the portion of the molecule that. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Soaps are cleaning. Soap Water Chemical Formula.
From www.scribd.com
formula of liquid soap.docx Chemical Substances Chemical Compounds Soap Water Chemical Formula Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. One the above structure, circle the portion of the molecule that. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both. The reaction produces sodium salts of.. Soap Water Chemical Formula.
From www.scribd.com
Soap Soap Chemical Substances Soap Water Chemical Formula Soap is a classic cleaning agent that has been used for centuries. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. One the above structure, circle the portion of the molecule that. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water. Soap Water Chemical Formula.
From www.tessshebaylo.com
Chemical Equation For Ionisation Of Detergent In Water Tessshebaylo Soap Water Chemical Formula Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. The reaction produces sodium salts of. Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty. Soap Water Chemical Formula.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Soap Water Chemical Formula The reaction produces sodium salts of. Soap is a classic cleaning agent that has been used for centuries. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our. Soap Water Chemical Formula.
From www.alamy.com
General formula of solid and liquid soap molecule. RCOONa, RCOOK Soap Water Chemical Formula Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Its fundamental chemistry involves the combination of fats or oils with. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. One the above structure, circle the portion. Soap Water Chemical Formula.
From www.slideserve.com
PPT Soap Describe how soap is made from fatty acids and alkalis Soap Water Chemical Formula Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. One the above structure, circle the portion of the molecule that. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty. Soap Water Chemical Formula.
From www.slideshare.net
Chemistry of soaps Soap Water Chemical Formula Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both. Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Soap is a classic cleaning agent that has been used for centuries. Soaps are cleaning agents that are usually made by reacting alkali (e.g.,. Soap Water Chemical Formula.
From www.chemxpertise.com
Liquid Soap Formula Chemxpertise Soap Water Chemical Formula Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Its fundamental chemistry involves the combination of fats or oils with. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. The reaction produces sodium salts of. Carboxylic acids and salts having alkyl chains longer. Soap Water Chemical Formula.
From ar.inspiredpencil.com
Soap Molecule Structure Soap Water Chemical Formula Its fundamental chemistry involves the combination of fats or oils with. Soap is a classic cleaning agent that has been used for centuries. The reaction produces sodium salts of. Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring. Soap Water Chemical Formula.
From www.pinterest.co.uk
Hand washing with soap vector illustration. Educational explanation Soap Water Chemical Formula Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. The reaction produces sodium salts of. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Soaps are cleaning agents. Soap Water Chemical Formula.
From spmchemistry.blog.onlinetuition.com.my
The Making of Soap Saponification SPM Chemistry Soap Water Chemical Formula Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. The reaction produces sodium salts of. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers. Soap Water Chemical Formula.
From ar.inspiredpencil.com
Soap Molecular Formula Soap Water Chemical Formula Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. One the above structure, circle the portion of the molecule that. Its fundamental chemistry involves the combination of fats or oils with. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Revise the. Soap Water Chemical Formula.
From www.slideserve.com
PPT Hardness of Water PowerPoint Presentation, free download ID2956417 Soap Water Chemical Formula Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Its fundamental chemistry involves the combination of fats or oils with. Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and. One the above structure, circle the portion of the molecule that. Revise the action of soaps and detergents for higher. Soap Water Chemical Formula.
From ar.inspiredpencil.com
Soap Molecule Structure Soap Water Chemical Formula Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. One the above structure, circle the portion of the molecule that. Its fundamental chemistry involves the combination of fats or oils with. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of. Soap Water Chemical Formula.
From cartoondealer.com
General Formula Of Solid And Liquid Soap Molecule. RCOONa, RCOOK Soap Water Chemical Formula One the above structure, circle the portion of the molecule that. The reaction produces sodium salts of. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Its fundamental chemistry involves the combination of fats or oils with. Soap is produced by a saponification or basic hydrolysis reaction of. Soap Water Chemical Formula.
From ar.inspiredpencil.com
Soap Molecule Polar Or Nonpolar Soap Water Chemical Formula Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. The reaction produces sodium salts of. Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both. Soaps are cleaning agents that are usually made by reacting alkali. Soap Water Chemical Formula.
From imgigi.com
liquid detergent formulation liquid detergent formulation pdf,liquid Soap Water Chemical Formula Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both. One the above structure, circle the portion of the molecule that. Its fundamental chemistry involves the combination of fats or oils with. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Revise. Soap Water Chemical Formula.