Indicator For Starch Titration . It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Information about end point detection with starch in iodometric titration. To prepare starch indicator solution, add 1 gram of starch (either. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Iodine in water solutions is usually colored strong. Starch solution is used for end point detection in iodometric titration. Ascorbic acid reacts with iodine to make dehydroascorbic acid and. Use a blank titration to correct the volume of titrant.
from slideplayer.com
Use a blank titration to correct the volume of titrant. Iodine in water solutions is usually colored strong. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Information about end point detection with starch in iodometric titration. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Starch solution is used for end point detection in iodometric titration. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. To prepare starch indicator solution, add 1 gram of starch (either. Ascorbic acid reacts with iodine to make dehydroascorbic acid and.
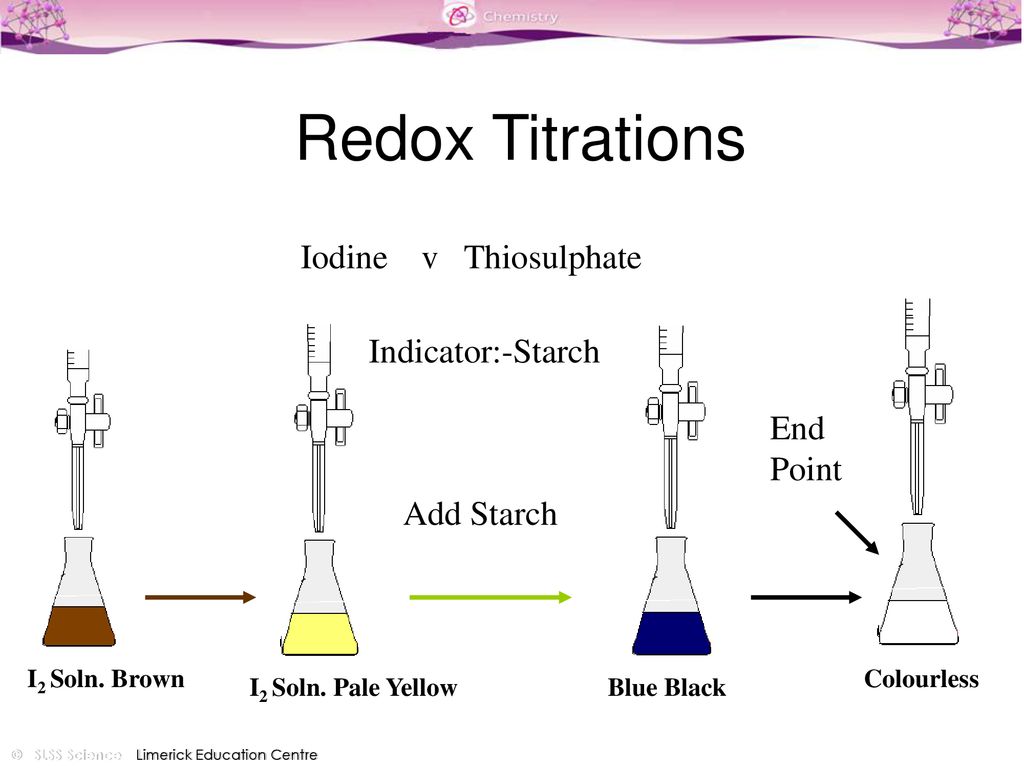
Titration Colour Changes ppt download
Indicator For Starch Titration Information about end point detection with starch in iodometric titration. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Starch solution is used for end point detection in iodometric titration. Information about end point detection with starch in iodometric titration. Ascorbic acid reacts with iodine to make dehydroascorbic acid and. To prepare starch indicator solution, add 1 gram of starch (either. Iodine in water solutions is usually colored strong. Use a blank titration to correct the volume of titrant. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be.
From www.fishersci.ca
Starch Indicator, 1 (w/v), Mercury Free, for Iodometric Titrations Indicator For Starch Titration Information about end point detection with starch in iodometric titration. Ascorbic acid reacts with iodine to make dehydroascorbic acid and. Starch solution is used for end point detection in iodometric titration. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. An example of titration using a starch indicator is the titration of vitamin c,. Indicator For Starch Titration.
From lamotte.com
Starch Acid Indicator Powder, 10G Titration Reagent Indicator For Starch Titration To prepare starch indicator solution, add 1 gram of starch (either. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Iodine in water solutions is usually colored strong. Use a blank titration to. Indicator For Starch Titration.
From education.seattlepi.com
What Is the Purpose of Adding Starch to the Titration Mixture Indicator For Starch Titration Ascorbic acid reacts with iodine to make dehydroascorbic acid and. Use a blank titration to correct the volume of titrant. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Starch solution is used for end point detection in iodometric titration. An example of titration using a starch indicator is the titration of. Indicator For Starch Titration.
From www.youtube.com
Preparation of Starch 1 indicator solution for titration YouTube Indicator For Starch Titration An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Use a blank titration to correct the volume of titrant. Ascorbic acid reacts with iodine to make dehydroascorbic acid and. Iodine in water solutions is usually. Indicator For Starch Titration.
From dokumen.tips
(PDF) Indicator.pdf DOKUMEN.TIPS Indicator For Starch Titration An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Starch solution is used for end point detection in iodometric titration. Iodine in water solutions is usually colored strong. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodometric titration is a method used to. Indicator For Starch Titration.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Indicator For Starch Titration An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Ascorbic acid reacts with iodine to make dehydroascorbic acid. Indicator For Starch Titration.
From lulelaboratory.blogspot.com
Lu Le Laboratory August 2013 Indicator For Starch Titration It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodine in water solutions is usually colored strong. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. To prepare starch indicator solution, add 1 gram of starch (either. Information about end point detection with starch. Indicator For Starch Titration.
From www.chemiepedia.nl
Zuurbase titraties principe, soorten, toepassingen en procedures Indicator For Starch Titration Iodine in water solutions is usually colored strong. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Information about end point detection with starch in iodometric titration. To prepare starch indicator solution, add 1 gram of starch (either. Iodometric titration is a method used to measure the amount of an. Indicator For Starch Titration.
From www.chemedx.org
Determination of Vitamin C in a Produce Protector Iodometric Method Indicator For Starch Titration Iodine in water solutions is usually colored strong. Starch solution is used for end point detection in iodometric titration. Ascorbic acid reacts with iodine to make dehydroascorbic acid and. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodometric titration is a method used to measure the amount of an oxidizing agent in a. Indicator For Starch Titration.
From www.youtube.com
Starch Indicator YouTube Indicator For Starch Titration Ascorbic acid reacts with iodine to make dehydroascorbic acid and. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Use a blank titration to correct the volume of titrant. To prepare starch indicator solution, add 1 gram of starch (either. Starch solution is used for end point detection in iodometric. Indicator For Starch Titration.
From vinmetrica.com
RRS Starch Indicator Vinmetrica Sulfite (SO2), Malic, Alcohol & pH Indicator For Starch Titration Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Use a blank titration to correct the volume of titrant. Iodine in water solutions is usually colored strong. Ascorbic acid reacts with iodine to make dehydroascorbic acid and. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be.. Indicator For Starch Titration.
From cymitquimica.com
Reagecon Starch Indicator 1 Solution CymitQuimica Indicator For Starch Titration It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. To prepare starch indicator solution, add 1 gram of starch (either. Starch solution is used for end point detection in iodometric titration. Use a blank titration to correct the volume of titrant. Iodine in water solutions is usually colored strong. Iodometric titration is a method. Indicator For Starch Titration.
From www.reddit.com
Titration using starch as an indicator for Iodine! At the end, one drop Indicator For Starch Titration An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Iodine in water solutions is usually colored strong. Information about end point detection with starch in iodometric titration. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Starch solution is used for end point detection. Indicator For Starch Titration.
From hxejxcnlz.blob.core.windows.net
Indicator Use In Lodimetric Titration at Patricia Jolley blog Indicator For Starch Titration Ascorbic acid reacts with iodine to make dehydroascorbic acid and. Starch solution is used for end point detection in iodometric titration. Use a blank titration to correct the volume of titrant. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Iodometric titration is a method used to measure the amount. Indicator For Starch Titration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Indicator For Starch Titration Use a blank titration to correct the volume of titrant. Ascorbic acid reacts with iodine to make dehydroascorbic acid and. Starch solution is used for end point detection in iodometric titration. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Information about end point detection with starch in iodometric titration. Iodine in water solutions. Indicator For Starch Titration.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Indicator For Starch Titration Iodine in water solutions is usually colored strong. To prepare starch indicator solution, add 1 gram of starch (either. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Use a blank titration to correct the volume of titrant. Information about end point detection with starch in iodometric titration. An example of titration using a. Indicator For Starch Titration.
From mirjamglessmer.com
Measuring the concentration of dissolved oxygen in sea water Part 3 Indicator For Starch Titration Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Information about end point detection with starch in iodometric titration. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Use a blank titration to correct the volume of titrant. Starch solution is used. Indicator For Starch Titration.
From www.numerade.com
SOLVED IODOMETRIC TITRATION (II) ANALYSIS OF UNKNOWN SAMPLE APPARATUS Indicator For Starch Titration Information about end point detection with starch in iodometric titration. Ascorbic acid reacts with iodine to make dehydroascorbic acid and. Use a blank titration to correct the volume of titrant. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Starch solution is used for end point detection in iodometric titration. To prepare starch indicator. Indicator For Starch Titration.
From www.amazon.com
ALDON Innovating Science 1 Starch Indicator Solution, 1L Indicator For Starch Titration Iodine in water solutions is usually colored strong. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Information. Indicator For Starch Titration.
From slideplayer.com
Titration Colour Changes ppt download Indicator For Starch Titration Iodine in water solutions is usually colored strong. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. To prepare starch indicator solution, add 1 gram of starch (either. Iodometric titration is a method used to. Indicator For Starch Titration.
From www.numerade.com
SOLVED(A) Starch is the indicator used in the iodometric titrations Indicator For Starch Titration An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Use a blank titration to correct the volume of titrant. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. To prepare starch indicator solution, add 1 gram of starch (either. Information about end point detection. Indicator For Starch Titration.
From maddoxkruwbridges.blogspot.com
What Chemical Is Used to Test for Starch MaddoxkruwBridges Indicator For Starch Titration Use a blank titration to correct the volume of titrant. Iodine in water solutions is usually colored strong. Starch solution is used for end point detection in iodometric titration. Information about end point detection with starch in iodometric titration. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. An example of titration. Indicator For Starch Titration.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Volumetric Analysis (3) Redox Titrations Indicator For Starch Titration Iodine in water solutions is usually colored strong. Use a blank titration to correct the volume of titrant. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Starch solution is used for end point detection in iodometric titration. To prepare starch indicator solution, add 1 gram of starch (either. An example of. Indicator For Starch Titration.
From mungfali.com
Acid Base Titration Indicator Indicator For Starch Titration Information about end point detection with starch in iodometric titration. Ascorbic acid reacts with iodine to make dehydroascorbic acid and. Iodine in water solutions is usually colored strong. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Starch solution is used for end point detection in iodometric titration. Use a. Indicator For Starch Titration.
From www.toppr.com
Match the titrations given with the indicators used in them Titration Indicator For Starch Titration Ascorbic acid reacts with iodine to make dehydroascorbic acid and. Starch solution is used for end point detection in iodometric titration. Iodine in water solutions is usually colored strong. Use a blank titration to correct the volume of titrant. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. To prepare starch indicator solution, add. Indicator For Starch Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator For Starch Titration Ascorbic acid reacts with iodine to make dehydroascorbic acid and. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. To prepare starch indicator solution, add 1 gram of starch (either. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Starch solution is used for. Indicator For Starch Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator For Starch Titration Ascorbic acid reacts with iodine to make dehydroascorbic acid and. Information about end point detection with starch in iodometric titration. To prepare starch indicator solution, add 1 gram of starch (either. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Use a blank titration to correct the volume of titrant. Starch solution. Indicator For Starch Titration.
From studylib.net
Prelab What is a titration? Why is the starch indicator added near Indicator For Starch Titration Information about end point detection with starch in iodometric titration. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. To prepare starch indicator solution, add 1 gram of starch (either. Ascorbic acid reacts with iodine to make. Indicator For Starch Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator For Starch Titration Ascorbic acid reacts with iodine to make dehydroascorbic acid and. To prepare starch indicator solution, add 1 gram of starch (either. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Information about end point detection with starch in iodometric titration. Iodometric titration is a method used to measure the amount of an oxidizing agent. Indicator For Starch Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator For Starch Titration Use a blank titration to correct the volume of titrant. Iodine in water solutions is usually colored strong. Ascorbic acid reacts with iodine to make dehydroascorbic acid and. Information about end point detection with starch in iodometric titration. Starch solution is used for end point detection in iodometric titration. Iodometric titration is a method used to measure the amount of. Indicator For Starch Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator For Starch Titration Iodine in water solutions is usually colored strong. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. To prepare starch indicator solution, add 1 gram of starch (either. Use a blank titration to correct the volume of titrant. Starch solution is used for end point detection in iodometric titration. Information. Indicator For Starch Titration.
From fphoto.photoshelter.com
science chemistry oxidation reaction starch iodine Fundamental Indicator For Starch Titration It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodine in water solutions is usually colored strong. Information about end point detection with starch in iodometric titration. Starch solution is used for end point detection in iodometric titration. To prepare starch indicator solution, add 1 gram of starch (either. Use a blank titration to. Indicator For Starch Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Indicator For Starch Titration Iodine in water solutions is usually colored strong. Information about end point detection with starch in iodometric titration. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Ascorbic acid reacts with iodine to make dehydroascorbic acid and. To prepare starch indicator solution, add 1 gram of starch (either. It works by mixing. Indicator For Starch Titration.
From www.youtube.com
How to prepare starch indicator starch indicator preparation how to Indicator For Starch Titration It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. To prepare starch indicator solution, add 1 gram of starch (either. Iodine in water solutions is usually colored strong. Information about end point detection with starch. Indicator For Starch Titration.
From studyespacioec4o.z22.web.core.windows.net
How To Do Titrations In Chemistry Indicator For Starch Titration Starch solution is used for end point detection in iodometric titration. An example of titration using a starch indicator is the titration of vitamin c, which is technically ascorbic acid. Information about end point detection with starch in iodometric titration. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. To prepare starch indicator solution,. Indicator For Starch Titration.