How Are Water Molecules Bonded To Vanadium Ion . The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. The density of ice is less than water. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Metals in general will form complexes with water, through coordinate bonds with the an. (b) vanadium (iii) complexes can be used as catalysts. The compounds of vanadium reflect the varied oxidation states possible for this element. The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. This is due to the water expanding as it is frozen because. Water has some unusual properties due to the hydrogen bonding between its molecules. Formal oxidation states of +5 to −1 have been found, with. Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to a. Vanadate, vanadyl, and vanadium(iii) ions in acidic water.
from mungfali.com
Formal oxidation states of +5 to −1 have been found, with. This is due to the water expanding as it is frozen because. Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to a. The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. The compounds of vanadium reflect the varied oxidation states possible for this element. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Water has some unusual properties due to the hydrogen bonding between its molecules. (b) vanadium (iii) complexes can be used as catalysts. Vanadate, vanadyl, and vanadium(iii) ions in acidic water. The density of ice is less than water.
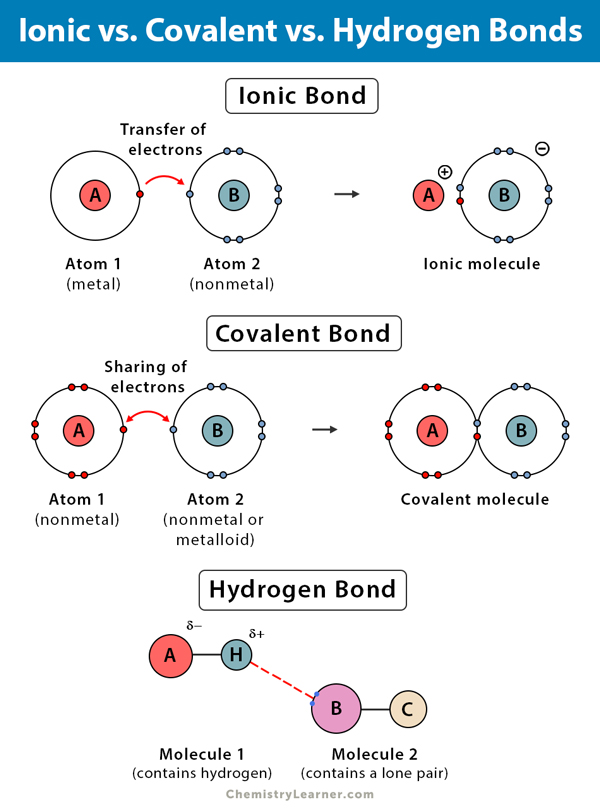
Water Molecules Covalent And Hydrogen Bonds
How Are Water Molecules Bonded To Vanadium Ion (b) vanadium (iii) complexes can be used as catalysts. Water has some unusual properties due to the hydrogen bonding between its molecules. Formal oxidation states of +5 to −1 have been found, with. Vanadate, vanadyl, and vanadium(iii) ions in acidic water. Metals in general will form complexes with water, through coordinate bonds with the an. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. This is due to the water expanding as it is frozen because. The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. The compounds of vanadium reflect the varied oxidation states possible for this element. Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to a. The density of ice is less than water. The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. (b) vanadium (iii) complexes can be used as catalysts.
From www.science-revision.co.uk
Covalent bonding How Are Water Molecules Bonded To Vanadium Ion The compounds of vanadium reflect the varied oxidation states possible for this element. The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to a. In water, each hydrogen nucleus is covalently bound. How Are Water Molecules Bonded To Vanadium Ion.
From mungfali.com
Water Molecules Covalent And Hydrogen Bonds How Are Water Molecules Bonded To Vanadium Ion In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. (b) vanadium (iii) complexes can be used as catalysts. Water has some unusual properties due to the hydrogen bonding between its molecules. The binding energies of water molecules to central vanadium cations is calculated for the above. How Are Water Molecules Bonded To Vanadium Ion.
From www.youtube.com
How many hydrogen bonds a water molecule can form Hydrogen Bonding in How Are Water Molecules Bonded To Vanadium Ion The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. Water has some unusual properties due to the hydrogen bonding between its molecules. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Metals in general. How Are Water Molecules Bonded To Vanadium Ion.
From www.sciencephoto.com
Bond formation in water molecule Stock Image C028/6477 Science How Are Water Molecules Bonded To Vanadium Ion Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to a. The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. Metals in general will form complexes with water, through coordinate bonds with the an. In water, each hydrogen nucleus is covalently bound to. How Are Water Molecules Bonded To Vanadium Ion.
From www.slideserve.com
PPT Water Chemistry & Properties of Water PowerPoint Presentation How Are Water Molecules Bonded To Vanadium Ion Formal oxidation states of +5 to −1 have been found, with. The density of ice is less than water. Vanadate, vanadyl, and vanadium(iii) ions in acidic water. The compounds of vanadium reflect the varied oxidation states possible for this element. Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to a. Metals in general will. How Are Water Molecules Bonded To Vanadium Ion.
From pubs.acs.org
A Coordination Chemistry Study of Hydrated and Solvated Cationic How Are Water Molecules Bonded To Vanadium Ion Metals in general will form complexes with water, through coordinate bonds with the an. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Vanadate, vanadyl, and vanadium(iii) ions in acidic water. The electronic structure of the complex depends strongly on the mode of coordination of the. How Are Water Molecules Bonded To Vanadium Ion.
From exyjskkyy.blob.core.windows.net
What Enzyme Breaks Down Hydrogen Bonds at Bobby Wilkerson blog How Are Water Molecules Bonded To Vanadium Ion Metals in general will form complexes with water, through coordinate bonds with the an. Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to a. The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. The electronic structure of the complex depends strongly on. How Are Water Molecules Bonded To Vanadium Ion.
From wiki.answers.com
Hydrogen bonding in water is it a covalent bond or ionic bond How Are Water Molecules Bonded To Vanadium Ion Formal oxidation states of +5 to −1 have been found, with. The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. (b) vanadium (iii) complexes can be used as catalysts. Metals in general will form complexes with water, through coordinate bonds with the an. The binding energies. How Are Water Molecules Bonded To Vanadium Ion.
From scienceatyourdoorstep.com
Types of Atoms Science at Your Doorstep How Are Water Molecules Bonded To Vanadium Ion This is due to the water expanding as it is frozen because. The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. The compounds of vanadium reflect the varied oxidation states possible for this element. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by. How Are Water Molecules Bonded To Vanadium Ion.
From quizlet.com
hydrogen bond between water molecules Diagram Quizlet How Are Water Molecules Bonded To Vanadium Ion Formal oxidation states of +5 to −1 have been found, with. The compounds of vanadium reflect the varied oxidation states possible for this element. This is due to the water expanding as it is frozen because. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Figure. How Are Water Molecules Bonded To Vanadium Ion.
From www.pinterest.com.au
Water has both a hydrogen bond and a polar covalent bond. Hydrogen How Are Water Molecules Bonded To Vanadium Ion Metals in general will form complexes with water, through coordinate bonds with the an. Water has some unusual properties due to the hydrogen bonding between its molecules. This is due to the water expanding as it is frozen because. The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii). How Are Water Molecules Bonded To Vanadium Ion.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts How Are Water Molecules Bonded To Vanadium Ion (b) vanadium (iii) complexes can be used as catalysts. Metals in general will form complexes with water, through coordinate bonds with the an. The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. Vanadate, vanadyl, and vanadium(iii) ions in acidic water. The electronic structure of the complex depends strongly. How Are Water Molecules Bonded To Vanadium Ion.
From klaszcmjb.blob.core.windows.net
How Is Water Bonded Together at Anthony Faison blog How Are Water Molecules Bonded To Vanadium Ion Formal oxidation states of +5 to −1 have been found, with. The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. Metals in general will form complexes with water, through coordinate bonds with the an. The electronic structure of the complex depends strongly on the mode of coordination of. How Are Water Molecules Bonded To Vanadium Ion.
From quizlet.com
Given figure illustrates five water molecules held together Quizlet How Are Water Molecules Bonded To Vanadium Ion Formal oxidation states of +5 to −1 have been found, with. This is due to the water expanding as it is frozen because. The density of ice is less than water. The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. Water has some unusual properties due to the. How Are Water Molecules Bonded To Vanadium Ion.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica How Are Water Molecules Bonded To Vanadium Ion Formal oxidation states of +5 to −1 have been found, with. (b) vanadium (iii) complexes can be used as catalysts. The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. The binding energies of water molecules to central vanadium cations is calculated for the above structures and. How Are Water Molecules Bonded To Vanadium Ion.
From essenceofwater.org
Structure of Water Essence of Water Essence of Water How Are Water Molecules Bonded To Vanadium Ion Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to a. Formal oxidation states of +5 to −1 have been found, with. The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. The density of ice is less than water. In water, each hydrogen. How Are Water Molecules Bonded To Vanadium Ion.
From chem.libretexts.org
24.5 Bonding in Complex Ions Crystal Field Theory Chemistry LibreTexts How Are Water Molecules Bonded To Vanadium Ion The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. Metals in general will form complexes with water, through coordinate bonds with the an. The compounds of vanadium reflect the varied oxidation states possible for this element. Vanadate, vanadyl, and vanadium(iii) ions in acidic water. In water, each hydrogen. How Are Water Molecules Bonded To Vanadium Ion.
From surfguppy.com
What is Polar Covalent Bond How Are Water Molecules Bonded To Vanadium Ion Vanadate, vanadyl, and vanadium(iii) ions in acidic water. The compounds of vanadium reflect the varied oxidation states possible for this element. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. The binding energies of water molecules to central vanadium cations is calculated for the above structures. How Are Water Molecules Bonded To Vanadium Ion.
From www.gauthmath.com
The diagram below illustrates a network of water molecules bonded How Are Water Molecules Bonded To Vanadium Ion Formal oxidation states of +5 to −1 have been found, with. The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. Vanadate, vanadyl, and vanadium(iii) ions in acidic water. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons. How Are Water Molecules Bonded To Vanadium Ion.
From slidetodoc.com
The chemistry of vanadium Variable Oxidation States What How Are Water Molecules Bonded To Vanadium Ion This is due to the water expanding as it is frozen because. The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. Formal oxidation states of +5 to −1 have been found, with. The density of ice is less than water. Water has some unusual properties due. How Are Water Molecules Bonded To Vanadium Ion.
From www.acs.org
Lesson 5.1 Water is a Polar Molecule American Chemical Society How Are Water Molecules Bonded To Vanadium Ion The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. This is due to the water expanding as it is frozen because. Water has some unusual properties due to the hydrogen bonding between its molecules. Formal oxidation states of +5 to −1 have been found, with. The electronic structure. How Are Water Molecules Bonded To Vanadium Ion.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID How Are Water Molecules Bonded To Vanadium Ion The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. Water has some unusual properties due to the hydrogen bonding between its molecules. Metals in general will form complexes with water, through coordinate bonds with the an. This is due to the water expanding as it is. How Are Water Molecules Bonded To Vanadium Ion.
From brainly.com
Draw a diagram of water molecules, labeling the hydrogen bond and How Are Water Molecules Bonded To Vanadium Ion The compounds of vanadium reflect the varied oxidation states possible for this element. Formal oxidation states of +5 to −1 have been found, with. Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to a. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are. How Are Water Molecules Bonded To Vanadium Ion.
From cmapsconverted.ihmc.us
water_practical molecules_of_life How Are Water Molecules Bonded To Vanadium Ion The compounds of vanadium reflect the varied oxidation states possible for this element. Metals in general will form complexes with water, through coordinate bonds with the an. The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. The binding energies of water molecules to central vanadium cations. How Are Water Molecules Bonded To Vanadium Ion.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica How Are Water Molecules Bonded To Vanadium Ion The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. Water has some unusual properties due to the hydrogen bonding between its molecules. The density of ice is less than water. Formal oxidation states of +5 to −1 have been found, with. Figure 2 shows a vanadium. How Are Water Molecules Bonded To Vanadium Ion.
From chemistryskills.com
Hydrogen Bonding Chemistry Skills How Are Water Molecules Bonded To Vanadium Ion Formal oxidation states of +5 to −1 have been found, with. Water has some unusual properties due to the hydrogen bonding between its molecules. The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table 4. The compounds of vanadium reflect the varied oxidation states possible for this element. Metals in. How Are Water Molecules Bonded To Vanadium Ion.
From www3.nd.edu
Hydrogen bond between two water molecules How Are Water Molecules Bonded To Vanadium Ion Water has some unusual properties due to the hydrogen bonding between its molecules. The compounds of vanadium reflect the varied oxidation states possible for this element. Metals in general will form complexes with water, through coordinate bonds with the an. The binding energies of water molecules to central vanadium cations is calculated for the above structures and presented in table. How Are Water Molecules Bonded To Vanadium Ion.
From owlcation.com
Primary and Secondary Bonds Owlcation How Are Water Molecules Bonded To Vanadium Ion Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to a. The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared. How Are Water Molecules Bonded To Vanadium Ion.
From www.pinterest.com
Why Is Water a Polar Molecule? Water molecule, Molecular geometry How Are Water Molecules Bonded To Vanadium Ion (b) vanadium (iii) complexes can be used as catalysts. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. This is due to the water expanding as it is frozen because. Vanadate, vanadyl, and vanadium(iii) ions in acidic water. Metals in general will form complexes with water,. How Are Water Molecules Bonded To Vanadium Ion.
From www.expii.com
Water — Molecular Structure & Bonding Expii How Are Water Molecules Bonded To Vanadium Ion Formal oxidation states of +5 to −1 have been found, with. Water has some unusual properties due to the hydrogen bonding between its molecules. The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. The compounds of vanadium reflect the varied oxidation states possible for this element.. How Are Water Molecules Bonded To Vanadium Ion.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Water and pH How Are Water Molecules Bonded To Vanadium Ion Vanadate, vanadyl, and vanadium(iii) ions in acidic water. The electronic structure of the complex depends strongly on the mode of coordination of the water molecules to the vanadium (iii) cation, as. Metals in general will form complexes with water, through coordinate bonds with the an. Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to. How Are Water Molecules Bonded To Vanadium Ion.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica How Are Water Molecules Bonded To Vanadium Ion In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. This is due to the water expanding as it is frozen because. Metals in general will form complexes with water, through coordinate bonds with the an. Vanadate, vanadyl, and vanadium(iii) ions in acidic water. Water has some. How Are Water Molecules Bonded To Vanadium Ion.
From drmchemistrytutor.com
Hydrogen Bonding in water Dr. M. Chemistry Tutor How Are Water Molecules Bonded To Vanadium Ion Formal oxidation states of +5 to −1 have been found, with. Metals in general will form complexes with water, through coordinate bonds with the an. (b) vanadium (iii) complexes can be used as catalysts. Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to a. This is due to the water expanding as it is. How Are Water Molecules Bonded To Vanadium Ion.
From techiescientist.com
Is H2O Ionic or Covalent? Techiescientist How Are Water Molecules Bonded To Vanadium Ion In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. The density of ice is less than water. Formal oxidation states of +5 to −1 have been found, with. Metals in general will form complexes with water, through coordinate bonds with the an. The binding energies of. How Are Water Molecules Bonded To Vanadium Ion.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts How Are Water Molecules Bonded To Vanadium Ion In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Metals in general will form complexes with water, through coordinate bonds with the an. The density of ice is less than water. Figure 2 shows a vanadium (iii) complex ion, where 6 water molecules are bonded to. How Are Water Molecules Bonded To Vanadium Ion.