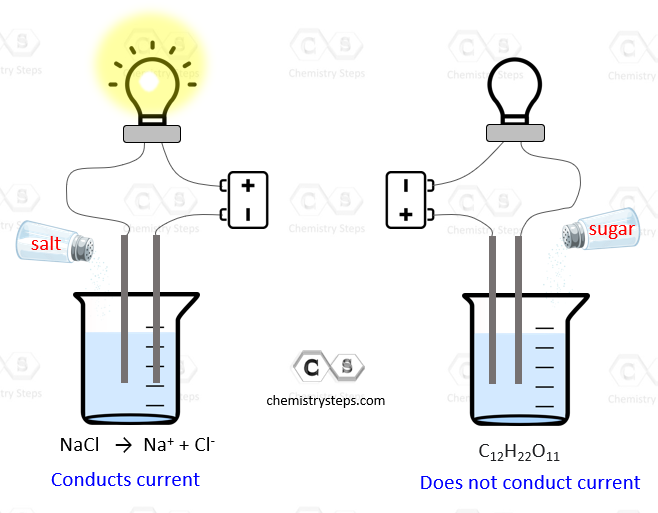
Is Distilled Water A Weak Electrolyte . in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. The solution will contain both ions and. weak electrolytes only partially break into ions in water. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Most compounds that contain nitrogen are weak electrolytes. while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or soil can be a source of. unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes, and many products contain. a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution.
from general.chemistrysteps.com
while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or soil can be a source of. a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. Most compounds that contain nitrogen are weak electrolytes. a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. weak electrolytes only partially break into ions in water. unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes, and many products contain. The solution will contain both ions and. in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. Weak electrolytes include weak acids, weak bases, and a variety of other compounds.
General Properties of Solutions Chemistry Steps
Is Distilled Water A Weak Electrolyte The solution will contain both ions and. a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Most compounds that contain nitrogen are weak electrolytes. a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes, and many products contain. while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or soil can be a source of. The solution will contain both ions and. in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. weak electrolytes only partially break into ions in water.
From www.shutterstock.com
19 Strong Electrolyte And Weak Electrolyte Images, Stock Photos Is Distilled Water A Weak Electrolyte a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes, and many products contain. in comparison,. Is Distilled Water A Weak Electrolyte.
From www.slideserve.com
PPT II. Types of Electrolytes PowerPoint Presentation, free download Is Distilled Water A Weak Electrolyte The solution will contain both ions and. a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes, and many products contain. Most compounds that contain nitrogen are weak electrolytes. in comparison,. Is Distilled Water A Weak Electrolyte.
From melissa-media.blogspot.com
Melissa Media Strong And Weak Electrolytes Is Distilled Water A Weak Electrolyte while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or soil can be a source of. weak electrolytes only partially break into ions in water. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. unless it’s labeled “ distilled,” your regular. Is Distilled Water A Weak Electrolyte.
From nataliaghopgrimes.blogspot.com
Why Is Tap Water a Weak Electrolyte Is Distilled Water A Weak Electrolyte a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes, and many products contain. weak electrolytes only partially break into ions in water. in comparison, distilled water is a very. Is Distilled Water A Weak Electrolyte.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Is Distilled Water A Weak Electrolyte a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. The solution will contain both ions and. a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. weak electrolytes only partially break into ions in water. unless it’s labeled “ distilled,” your regular. Is Distilled Water A Weak Electrolyte.
From quizdbbarnstorms.z21.web.core.windows.net
What Is Electrolyte And Nonelectrolyte Is Distilled Water A Weak Electrolyte weak electrolytes only partially break into ions in water. a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. The solution will contain both ions and. while pure and distilled water is devoid. Is Distilled Water A Weak Electrolyte.
From circuitdbhomemade.z13.web.core.windows.net
Kcl Dissolved In Water Diagram Is Distilled Water A Weak Electrolyte a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. The solution will contain both ions and. a weak electrolyte is a. Is Distilled Water A Weak Electrolyte.
From quenchwater.com
Distilled Water vs Purified Water Quench Water Is Distilled Water A Weak Electrolyte unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes, and many products contain. The solution will contain both ions and. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Most compounds that contain nitrogen are weak electrolytes. weak electrolytes only partially break into ions in water.. Is Distilled Water A Weak Electrolyte.
From www.numerade.com
SOLVED Why must electrodes on the conductivity apparatus be rinsed Is Distilled Water A Weak Electrolyte while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or soil can be a source of. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. weak electrolytes only partially break into ions in water. unless it’s labeled “ distilled,” your regular. Is Distilled Water A Weak Electrolyte.
From minhkhuetravel.com
Is H2O A Strong Electrolyte Unveiling Its Conductivity Secrets Is Distilled Water A Weak Electrolyte Weak electrolytes include weak acids, weak bases, and a variety of other compounds. a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. weak electrolytes only partially break into ions in water. while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks. Is Distilled Water A Weak Electrolyte.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Is Distilled Water A Weak Electrolyte Weak electrolytes include weak acids, weak bases, and a variety of other compounds. in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. while pure and distilled water is devoid of electrolytes, natural water that. Is Distilled Water A Weak Electrolyte.
From sydneydesnhfrye.blogspot.com
Why Is Tap Water a Weak Electrolyte Is Distilled Water A Weak Electrolyte unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes, and many products contain. a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. Most compounds that contain nitrogen are weak electrolytes. The solution will contain both ions and. Weak electrolytes include. Is Distilled Water A Weak Electrolyte.
From www.slideserve.com
PPT Post Lab Electrolytes PowerPoint Presentation, free download Is Distilled Water A Weak Electrolyte in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or soil can be a source of. Most compounds that contain nitrogen are weak electrolytes. a weak electrolyte. Is Distilled Water A Weak Electrolyte.
From www.numerade.com
SOLVED Write the equation for the reaction that forms the few (but Is Distilled Water A Weak Electrolyte The solution will contain both ions and. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. . Is Distilled Water A Weak Electrolyte.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free Is Distilled Water A Weak Electrolyte in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. The solution will contain both ions and. a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. while pure and distilled water is devoid of electrolytes, natural water that comes. Is Distilled Water A Weak Electrolyte.
From www.slideserve.com
PPT Water, Electrolytes, and Solutions PowerPoint Presentation, free Is Distilled Water A Weak Electrolyte in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. The solution will contain both ions and. Most compounds that contain nitrogen are weak electrolytes. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. a weak electrolyte is a solution in which only a small fraction. Is Distilled Water A Weak Electrolyte.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps Is Distilled Water A Weak Electrolyte weak electrolytes only partially break into ions in water. a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. The solution will contain both ions and. in comparison, distilled water is a very. Is Distilled Water A Weak Electrolyte.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Is Distilled Water A Weak Electrolyte while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or soil can be a source of. weak electrolytes only partially break into ions in water. The solution will contain both ions and. a weak electrolyte is a solution in which only a small fraction of. Is Distilled Water A Weak Electrolyte.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free Is Distilled Water A Weak Electrolyte a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes, and many products contain. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. a weak electrolyte is a solution in which only. Is Distilled Water A Weak Electrolyte.
From gamesmartz.com
Weak Electrolyte Definition & Image GameSmartz Is Distilled Water A Weak Electrolyte while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or soil can be a source of. The solution will contain both ions and. Most compounds that contain nitrogen are weak electrolytes. unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount. Is Distilled Water A Weak Electrolyte.
From www.numerade.com
SOLVED POSTLABORATORY ASSIGNMENT Why is distilled water a weaker Is Distilled Water A Weak Electrolyte while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or soil can be a source of. in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. Weak electrolytes include weak acids, weak bases, and a variety of other. Is Distilled Water A Weak Electrolyte.
From www.yourdictionary.com
Examples of Electrolytes Basic Explanation and Purpose YourDictionary Is Distilled Water A Weak Electrolyte The solution will contain both ions and. weak electrolytes only partially break into ions in water. a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or. Is Distilled Water A Weak Electrolyte.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID3839298 Is Distilled Water A Weak Electrolyte a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. The solution will contain both ions and. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. weak electrolytes only partially break into ions in water. Most compounds that contain nitrogen are weak electrolytes. in. Is Distilled Water A Weak Electrolyte.
From www.artofit.org
Symptoms of low electrolytes and how to debug them infographic Artofit Is Distilled Water A Weak Electrolyte Weak electrolytes include weak acids, weak bases, and a variety of other compounds. in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. Most compounds that contain nitrogen are weak electrolytes. The solution will contain both ions and. while pure and distilled water is devoid of electrolytes, natural water that. Is Distilled Water A Weak Electrolyte.
From jasmine-has-cisneros.blogspot.com
Why Is Tap Water a Weak Electrolyte JasminehasCisneros Is Distilled Water A Weak Electrolyte a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. Most compounds that contain nitrogen are weak electrolytes. The solution will contain both ions and. in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. weak electrolytes only partially break. Is Distilled Water A Weak Electrolyte.
From flixwater.com
Distilled Water With Electrolytes FlixWater Is Distilled Water A Weak Electrolyte Weak electrolytes include weak acids, weak bases, and a variety of other compounds. a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. weak electrolytes only partially break into ions in water. while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks. Is Distilled Water A Weak Electrolyte.
From www.w3schools.blog
Strong and weak electrolytes W3schools Is Distilled Water A Weak Electrolyte Most compounds that contain nitrogen are weak electrolytes. The solution will contain both ions and. while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or soil can be a source of. unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount. Is Distilled Water A Weak Electrolyte.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free Is Distilled Water A Weak Electrolyte The solution will contain both ions and. weak electrolytes only partially break into ions in water. a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. Most compounds that contain nitrogen are weak electrolytes. while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts. Is Distilled Water A Weak Electrolyte.
From www.aakash.ac.in
Electrolytes and Nonelectrolytes Types, Differences & Examples AESL Is Distilled Water A Weak Electrolyte in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes, and many products contain. The solution will contain both ions and. Weak electrolytes include weak acids, weak bases, and a variety of other. Is Distilled Water A Weak Electrolyte.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Is Distilled Water A Weak Electrolyte Weak electrolytes include weak acids, weak bases, and a variety of other compounds. while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or soil can be a source of. unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes,. Is Distilled Water A Weak Electrolyte.
From www.slideserve.com
PPT Aqueoussolution Reactions PowerPoint Presentation ID159152 Is Distilled Water A Weak Electrolyte Most compounds that contain nitrogen are weak electrolytes. while pure and distilled water is devoid of electrolytes, natural water that comes into contact with minerals and salts in rocks or soil can be a source of. a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. a weak electrolyte is a solution in. Is Distilled Water A Weak Electrolyte.
From www.slideserve.com
PPT Water, Electrolytes, and Solutions PowerPoint Presentation, free Is Distilled Water A Weak Electrolyte a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. Most compounds that contain nitrogen are weak electrolytes. unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes, and many products contain. weak electrolytes only partially break into ions in water.. Is Distilled Water A Weak Electrolyte.
From www.slideserve.com
PPT Chapter 4 Reactions in Aqueous Solutions PowerPoint Presentation Is Distilled Water A Weak Electrolyte unless it’s labeled “ distilled,” your regular bottled water provides at least a small amount of electrolytes, and many products contain. a weak electrolyte is a solution in which only a small fraction of the dissolved solute exists as ions. in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through. Is Distilled Water A Weak Electrolyte.
From slideplayer.com
Bellwork Tuesday Today we are learning about solutions. ppt download Is Distilled Water A Weak Electrolyte Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Most compounds that contain nitrogen are weak electrolytes. weak electrolytes only partially break into ions in water. The solution will contain both ions and. in comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. unless it’s. Is Distilled Water A Weak Electrolyte.
From www.kentchemistry.com
Electrolytes Is Distilled Water A Weak Electrolyte Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Most compounds that contain nitrogen are weak electrolytes. a weak electrolyte is an electrolyte that does not completely dissociate in aqueous solution. weak electrolytes only partially break into ions in water. unless it’s labeled “ distilled,” your regular bottled water provides at least a. Is Distilled Water A Weak Electrolyte.