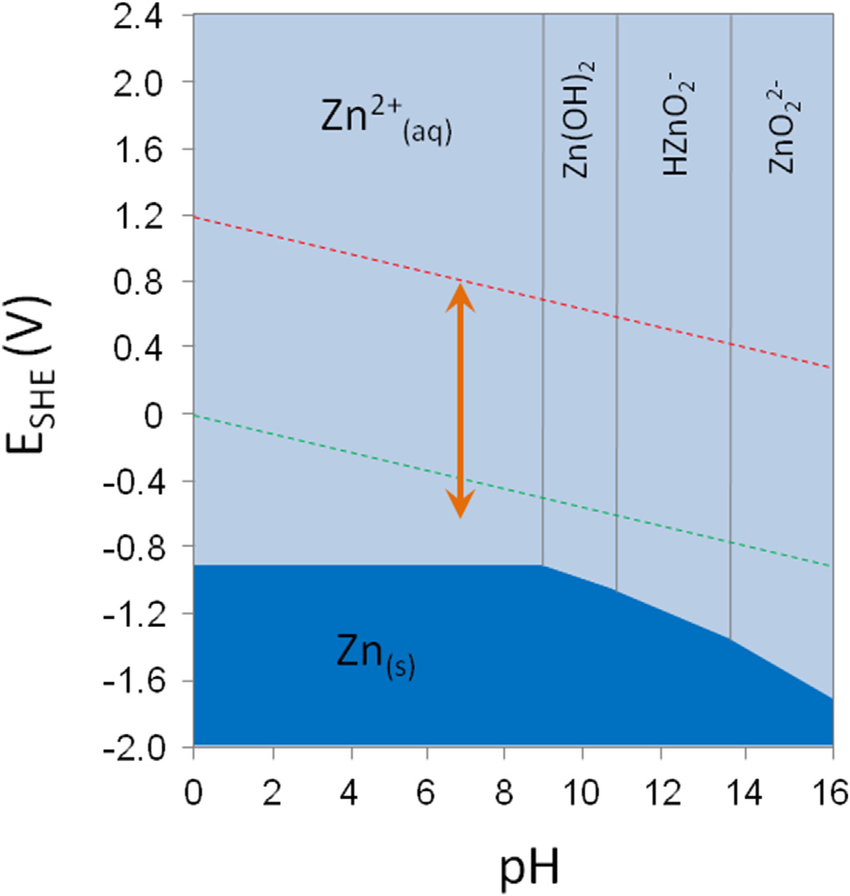
Zinc Hydroxide Is Soluble In Water . Zinc hydroxide reacts with both bases and acids. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. When excess sodium hydroxide is added to the. Hydroxide salts of transition metals and al 3+ are insoluble. It is an amphoteric hydroxide. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; When ammonia is added in excess, the. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2.
from socratic.org
Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble. It is an amphoteric hydroxide. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. Hydroxide salts of transition metals and al 3+ are insoluble. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Zinc hydroxide reacts with both bases and acids. When excess sodium hydroxide is added to the. It is an insoluble hydroxide which reacts with strong acid and gets dissolved.
How does zinc form an insoluble zinc hydroxide in water? Socratic
Zinc Hydroxide Is Soluble In Water It is an amphoteric hydroxide. Zinc hydroxide reacts with both bases and acids. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. When ammonia is added in excess, the. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; Hydroxide salts of transition metals and al 3+ are insoluble. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. When excess sodium hydroxide is added to the. It is an amphoteric hydroxide. Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble.
From www.numerade.com
SOLVED Consider the insoluble compound zinc hydroxide, Zn(OH)2. The zinc ion also forms a Zinc Hydroxide Is Soluble In Water Zinc hydroxide reacts with both bases and acids. When ammonia is added in excess, the. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Since. Zinc Hydroxide Is Soluble In Water.
From www.chegg.com
Solved Zinc hydroxide is only very s lightly soluble in Zinc Hydroxide Is Soluble In Water It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. When excess sodium hydroxide is added to the. Hydroxide salts of transition metals and al 3+ are insoluble. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. Since the ion is naturally surrounded. Zinc Hydroxide Is Soluble In Water.
From www.alamy.com
Zinc hydroxide precipitate formed by adding sodium hydroxide (NaOH) to a solution containing Zinc Hydroxide Is Soluble In Water Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. When excess sodium hydroxide is added to. Zinc Hydroxide Is Soluble In Water.
From whatsinsight.org
Zinc Hydroxide Structure, Properties, and Uses What's Insight Zinc Hydroxide Is Soluble In Water In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. When excess sodium hydroxide is added to the. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. It is an amphoteric hydroxide. Zinc hydroxide. Zinc Hydroxide Is Soluble In Water.
From slideplayer.com
YearLong Review Chemistry. ppt download Zinc Hydroxide Is Soluble In Water It is an amphoteric hydroxide. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; When ammonia is added in excess, the. Hydroxide salts of transition metals and al 3+ are insoluble. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3). Zinc Hydroxide Is Soluble In Water.
From ar.inspiredpencil.com
Zinc Hydroxide Zinc Hydroxide Is Soluble In Water Zinc hydroxide reacts with both bases and acids. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. It occurs. Zinc Hydroxide Is Soluble In Water.
From www.researchgate.net
The pH dependence of the solubility of zinc hydroxide (εZn(OH) 2(s) )... Download Scientific Zinc Hydroxide Is Soluble In Water On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble. When excess sodium hydroxide is added to the. It is an amphoteric hydroxide. Zinc hydroxide reacts with both bases and acids. When ammonia is added in excess, the. In general. Zinc Hydroxide Is Soluble In Water.
From advancedchemsys.com
Zinc Removal From Water Advanced Chemical Systems Zinc Hydroxide Is Soluble In Water Hydroxide salts of transition metals and al 3+ are insoluble. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. When. Zinc Hydroxide Is Soluble In Water.
From www.researchgate.net
The pH dependence of the solubility of zinc hydroxide (εZn(OH) 2(s) )... Download Scientific Zinc Hydroxide Is Soluble In Water It is an amphoteric hydroxide. Zinc hydroxide reacts with both bases and acids. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. When ammonia is added in excess, the. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble. When excess sodium. Zinc Hydroxide Is Soluble In Water.
From fineartamerica.com
Zinc Hydroxide Precipitate Photograph by Andrew Lambert Photography Zinc Hydroxide Is Soluble In Water Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; It is an amphoteric hydroxide. Hydroxide salts of transition metals and al 3+ are insoluble. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. Zinc hydroxide. Zinc Hydroxide Is Soluble In Water.
From socratic.org
How does zinc form an insoluble zinc hydroxide in water? Socratic Zinc Hydroxide Is Soluble In Water Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Hydroxide salts of transition metals and al 3+ are insoluble. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. Since the ion is. Zinc Hydroxide Is Soluble In Water.
From www.youtube.com
Zn+H2O=Zn(OH)2+H2 Balanced EquationZinc+Water=Zinc hydroxide+Water Balanced Equation YouTube Zinc Hydroxide Is Soluble In Water Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. It is an amphoteric hydroxide. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble. It occurs naturally in three rare minerals namely wulfingite, ashoverite and. Zinc Hydroxide Is Soluble In Water.
From www.youtube.com
Is ZnS Soluble or Insoluble in Water? YouTube Zinc Hydroxide Is Soluble In Water Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; It is an amphoteric hydroxide. Hydroxide salts of transition metals and al 3+ are insoluble. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Hydroxide salts of group ii elements (ca, sr, and ba) are slightly. Zinc Hydroxide Is Soluble In Water.
From www.youtube.com
17 5 7 Solubility of Zinc Hydroxide in 15 M NH3 11;54 YouTube Zinc Hydroxide Is Soluble In Water Zinc hydroxide reacts with both bases and acids. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. It is an amphoteric hydroxide. Hydroxide salts of transition metals and al 3+ are insoluble. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. Hydroxide salts of group ii elements (ca,. Zinc Hydroxide Is Soluble In Water.
From www.gkseries.com
What is formed when zinc reacts with sodium hydroxide? Zinc Hydroxide Is Soluble In Water It is an amphoteric hydroxide. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. When excess sodium hydroxide is added to the. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; It occurs naturally in three rare minerals namely wulfingite, ashoverite. Zinc Hydroxide Is Soluble In Water.
From www.slideserve.com
PPT Precipitation Equilibrium PowerPoint Presentation, free download ID6594495 Zinc Hydroxide Is Soluble In Water When excess sodium hydroxide is added to the. Hydroxide salts of transition metals and al 3+ are insoluble. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. Zinc hydroxide reacts with both bases. Zinc Hydroxide Is Soluble In Water.
From www.youtube.com
Solubility equilibrium Ksp zinc hydroxide YouTube Zinc Hydroxide Is Soluble In Water When ammonia is added in excess, the. Hydroxide salts of transition metals and al 3+ are insoluble. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; Hydroxide salts of group ii elements (ca,. Zinc Hydroxide Is Soluble In Water.
From woelen.homescience.net
Science made alive Chemistry/Solutions Zinc Hydroxide Is Soluble In Water Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; When excess sodium hydroxide is added to the. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Hydroxide salts of transition metals and. Zinc Hydroxide Is Soluble In Water.
From ar.inspiredpencil.com
Zinc Hydroxide Zinc Hydroxide Is Soluble In Water It is an amphoteric hydroxide. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Hydroxide salts of transition metals and al 3+ are insoluble. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is. Zinc Hydroxide Is Soluble In Water.
From www.researchgate.net
Simplified configuration of zinc reactions with water Download Scientific Diagram Zinc Hydroxide Is Soluble In Water When ammonia is added in excess, the. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. Zinc hydroxide reacts with both bases and acids. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound. Zinc Hydroxide Is Soluble In Water.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Zinc Hydroxide Is Soluble In Water Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. When ammonia is added in excess, the. Hydroxide salts of transition metals and al 3+ are insoluble. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; In general chemistry 1 students memorized a. Zinc Hydroxide Is Soluble In Water.
From www.dreamstime.com
Slow Process of Precipitation of White Insoluble Zinc Hydroxide Particles in Water, at the Zinc Hydroxide Is Soluble In Water Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble. When ammonia is added in excess, the. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. Hydroxide salts of transition metals and al 3+ are insoluble. On the solubility table we can see. Zinc Hydroxide Is Soluble In Water.
From www.youtube.com
Is Zn(OH)2 Soluble or Insoluble in Water? YouTube Zinc Hydroxide Is Soluble In Water Zinc hydroxide reacts with both bases and acids. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; It is an insoluble hydroxide which reacts with strong acid. Zinc Hydroxide Is Soluble In Water.
From www.chegg.com
Solved 2) Calculate the molar solubility of zinc hydroxide Zinc Hydroxide Is Soluble In Water In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; It is an amphoteric hydroxide. When excess sodium hydroxide is added to the. When ammonia. Zinc Hydroxide Is Soluble In Water.
From www.chegg.com
Solved 7. Solid Zinc Hydroxide, Zn(OH)dissolves in water to Zinc Hydroxide Is Soluble In Water Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. When ammonia is added in excess, the. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. It is an amphoteric hydroxide. It is an insoluble hydroxide which reacts with strong acid and gets. Zinc Hydroxide Is Soluble In Water.
From www.slideserve.com
PPT Terrence P. Sherlock Burlington County College 2004 PowerPoint Presentation ID4509695 Zinc Hydroxide Is Soluble In Water When excess sodium hydroxide is added to the. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. In general chemistry 1 students memorized a series of solubility rules (section. Zinc Hydroxide Is Soluble In Water.
From www.numerade.com
SOLVED 34. On the basis of the general solubility rules given in Table 4.1, predict which of Zinc Hydroxide Is Soluble In Water On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. Hydroxide salts of transition metals and al 3+ are insoluble. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; It is an amphoteric hydroxide. Zinc hydroxide reacts. Zinc Hydroxide Is Soluble In Water.
From www.youtube.com
Reaction of zinc sulphate (ZnSO4) with Ammonium hydroxide (NH4OH) YouTube Zinc Hydroxide Is Soluble In Water Zinc hydroxide reacts with both bases and acids. When ammonia is added in excess, the. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. It is an amphoteric hydroxide. When excess sodium hydroxide is added to the. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. On the solubility table we can see that. Zinc Hydroxide Is Soluble In Water.
From www.youtube.com
How to Balance Zn(OH)2 = ZnO + H2O (Zinc hydroxide YouTube Zinc Hydroxide Is Soluble In Water When excess sodium hydroxide is added to the. Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble. It is an amphoteric hydroxide. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Hydroxide salts of transition metals and al 3+ are insoluble. It is an insoluble. Zinc Hydroxide Is Soluble In Water.
From www.chegg.com
Solved Zinc hydroxide is sparingly soluble. Consider the Zinc Hydroxide Is Soluble In Water Zinc hydroxide reacts with both bases and acids. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. Hydroxide salts of transition metals and al 3+ are insoluble. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. When excess sodium hydroxide is added to the. It is an amphoteric. Zinc Hydroxide Is Soluble In Water.
From www.youtube.com
Zinc Powder in water YouTube Zinc Hydroxide Is Soluble In Water Hydroxide salts of transition metals and al 3+ are insoluble. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Zinc hydroxide reacts with both bases and acids. It is an amphoteric hydroxide. When ammonia is added in excess, the. When excess sodium hydroxide is added to the. On the. Zinc Hydroxide Is Soluble In Water.
From ar.inspiredpencil.com
Zinc Hydroxide Zinc Hydroxide Is Soluble In Water In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. It is an. Zinc Hydroxide Is Soluble In Water.
From www.gkseries.com
When sodium hydroxide reacts with Zinc it produces Zinc Hydroxide Is Soluble In Water Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble. It is an amphoteric hydroxide. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. Zinc hydroxide reacts with both bases and acids. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Hydroxide salts of. Zinc Hydroxide Is Soluble In Water.
From www.chegg.com
Solved Zinc hydroxide is sparingly soluble. Consider the Zinc Hydroxide Is Soluble In Water When excess sodium hydroxide is added to the. It is an amphoteric hydroxide. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Zinc hydroxide reacts with both bases and acids. Hydroxide salts of group ii elements (ca, sr, and ba) are slightly soluble. Hydroxide salts of transition metals and al 3+ are insoluble. It is an insoluble. Zinc Hydroxide Is Soluble In Water.
From www.youtube.com
How to write chemical formula of Zinc HydroxideMolecular formula of Zinc Hydroxide YouTube Zinc Hydroxide Is Soluble In Water It is an insoluble hydroxide which reacts with strong acid and gets dissolved. It is an amphoteric hydroxide. Hydroxide salts of transition metals and al 3+ are insoluble. On the solubility table we can see that zn (oh)2 (zinc hydroxide ) is insoluble in water. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict. Zinc Hydroxide Is Soluble In Water.