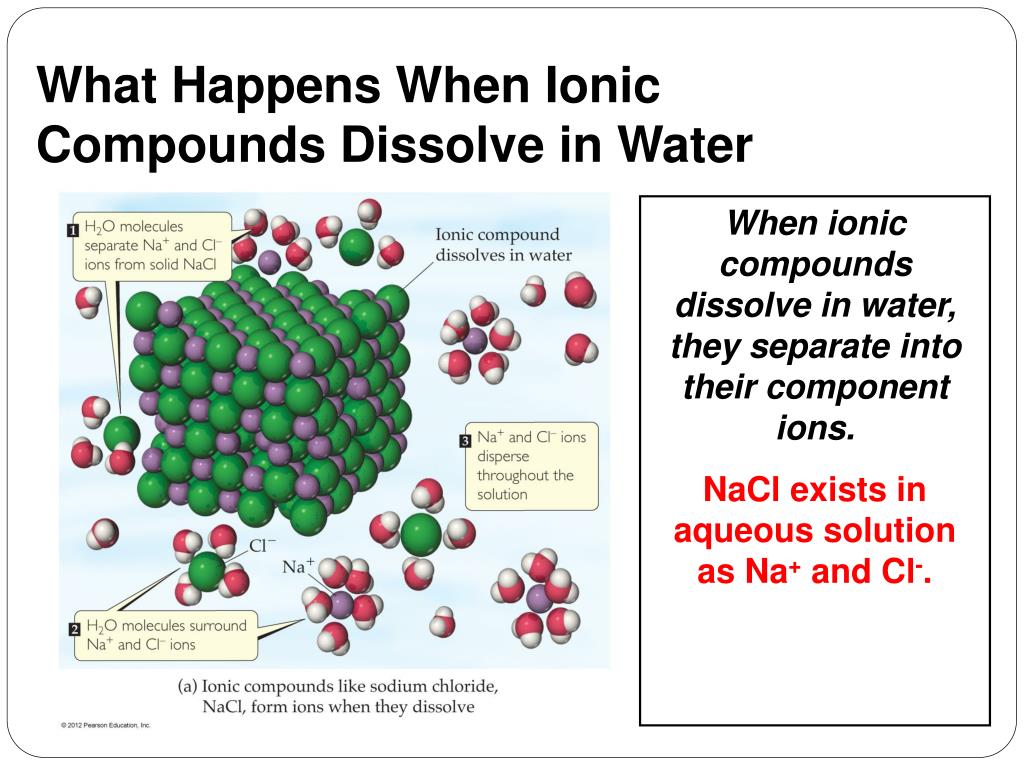
Which Ions Are Not Soluble In Water . salts of some halides are generally soluble. not all ionic compounds are soluble in water. substances that dissolve in water to yield ions are called electrolytes. A saturated solution is one in. Solubility rules can be used to predict whether a large number of ionic. some ions can be toxic when they separate in a solution but are helpful as part of a compound. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. Solubility is a result of an. this is a list of the solubility rules for ionic solids in water. substances that dissolve in water to yield ions are called electrolytes. The salts of silver (ag +),. Nonelectrolytes are substances that do not produce ions.
from www.slideserve.com
salts of some halides are generally soluble. Nonelectrolytes are substances that do not produce ions. not all ionic compounds are soluble in water. Solubility rules can be used to predict whether a large number of ionic. A saturated solution is one in. Solubility is a result of an. substances that dissolve in water to yield ions are called electrolytes. this is a list of the solubility rules for ionic solids in water. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. substances that dissolve in water to yield ions are called electrolytes.
PPT UNIT 5 PowerPoint Presentation, free download ID6635190
Which Ions Are Not Soluble In Water Nonelectrolytes are substances that do not produce ions. this is a list of the solubility rules for ionic solids in water. some ions can be toxic when they separate in a solution but are helpful as part of a compound. substances that dissolve in water to yield ions are called electrolytes. substances that dissolve in water to yield ions are called electrolytes. A saturated solution is one in. Nonelectrolytes are substances that do not produce ions. Solubility rules can be used to predict whether a large number of ionic. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. not all ionic compounds are soluble in water. Solubility is a result of an. salts of some halides are generally soluble. The salts of silver (ag +),.
From dxonxarcb.blob.core.windows.net
What Type Of Substances Will Dissolve In Water To Form Aqueous Which Ions Are Not Soluble In Water substances that dissolve in water to yield ions are called electrolytes. salts of some halides are generally soluble. A saturated solution is one in. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. some ions can be toxic when they separate in a solution but are helpful. Which Ions Are Not Soluble In Water.
From www.dexform.com
Solubility Rules Chart in Word and Pdf formats Which Ions Are Not Soluble In Water Nonelectrolytes are substances that do not produce ions. A saturated solution is one in. Solubility rules can be used to predict whether a large number of ionic. some ions can be toxic when they separate in a solution but are helpful as part of a compound. a solubility chart is a chart describing whether the ionic compounds formed. Which Ions Are Not Soluble In Water.
From www.chegg.com
Solved Some solubility rules for ionic compounds in water Which Ions Are Not Soluble In Water salts of some halides are generally soluble. substances that dissolve in water to yield ions are called electrolytes. A saturated solution is one in. substances that dissolve in water to yield ions are called electrolytes. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. this is. Which Ions Are Not Soluble In Water.
From ar.inspiredpencil.com
Solubility In Water Which Ions Are Not Soluble In Water substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. some ions can be toxic when they separate in a solution but are helpful as part of a compound. Solubility is a result of an. substances that dissolve in water to yield ions are called electrolytes. a. Which Ions Are Not Soluble In Water.
From exogctlqx.blob.core.windows.net
Examples Of Not Soluble In Water at Edward Brafford blog Which Ions Are Not Soluble In Water Solubility rules can be used to predict whether a large number of ionic. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. The salts of silver (ag +),. some ions can be toxic when they separate in a solution but are helpful as part of a compound. substances. Which Ions Are Not Soluble In Water.
From www.numerade.com
SOLVEDYou choose to investigate some of the solubility guidelines for Which Ions Are Not Soluble In Water The salts of silver (ag +),. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. salts of some halides are generally soluble. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. this is a list of the. Which Ions Are Not Soluble In Water.
From www.slideshare.net
Solubility rules and ionic equations 2 Which Ions Are Not Soluble In Water substances that dissolve in water to yield ions are called electrolytes. not all ionic compounds are soluble in water. A saturated solution is one in. this is a list of the solubility rules for ionic solids in water. substances that dissolve in water to yield ions are called electrolytes. some ions can be toxic when. Which Ions Are Not Soluble In Water.
From general.chemistrysteps.com
Dissociation of Ionic Compounds Chemistry Steps Which Ions Are Not Soluble In Water salts of some halides are generally soluble. The salts of silver (ag +),. substances that dissolve in water to yield ions are called electrolytes. A saturated solution is one in. this is a list of the solubility rules for ionic solids in water. Nonelectrolytes are substances that do not produce ions. some ions can be toxic. Which Ions Are Not Soluble In Water.
From exogctlqx.blob.core.windows.net
Examples Of Not Soluble In Water at Edward Brafford blog Which Ions Are Not Soluble In Water this is a list of the solubility rules for ionic solids in water. Solubility rules can be used to predict whether a large number of ionic. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. substances that dissolve in water to yield ions are called electrolytes. substances. Which Ions Are Not Soluble In Water.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Which Ions Are Not Soluble In Water this is a list of the solubility rules for ionic solids in water. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. Solubility is a result of an. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. . Which Ions Are Not Soluble In Water.
From www.numerade.com
SOLVED Ions are electrically neutral electrically charged formed by Which Ions Are Not Soluble In Water some ions can be toxic when they separate in a solution but are helpful as part of a compound. A saturated solution is one in. Nonelectrolytes are substances that do not produce ions. Solubility is a result of an. The salts of silver (ag +),. substances that dissolve in water to yield ions are called electrolytes. a. Which Ions Are Not Soluble In Water.
From exogctlqx.blob.core.windows.net
Examples Of Not Soluble In Water at Edward Brafford blog Which Ions Are Not Soluble In Water substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. The salts of silver (ag +),. Solubility is a. Which Ions Are Not Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Which Ions Are Not Soluble In Water Solubility rules can be used to predict whether a large number of ionic. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. salts of some halides are generally soluble. Nonelectrolytes are substances that do not produce ions. Solubility is a result of an. The salts of silver (ag +),.. Which Ions Are Not Soluble In Water.
From brentongokemckay.blogspot.com
What Happens When an Ionic Compound Dissolves in Water Which Ions Are Not Soluble In Water substances that dissolve in water to yield ions are called electrolytes. Solubility is a result of an. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. some ions can be toxic when they separate in a solution but are helpful as part of a compound. The salts of. Which Ions Are Not Soluble In Water.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint Which Ions Are Not Soluble In Water Solubility is a result of an. this is a list of the solubility rules for ionic solids in water. Solubility rules can be used to predict whether a large number of ionic. some ions can be toxic when they separate in a solution but are helpful as part of a compound. The salts of silver (ag +),. . Which Ions Are Not Soluble In Water.
From www.numerade.com
SOLVED Using the solubility table below, determine if sodium sulfate Which Ions Are Not Soluble In Water Nonelectrolytes are substances that do not produce ions. A saturated solution is one in. not all ionic compounds are soluble in water. Solubility rules can be used to predict whether a large number of ionic. salts of some halides are generally soluble. substances that dissolve in water to yield ions are called electrolytes. some ions can. Which Ions Are Not Soluble In Water.
From www.slideserve.com
PPT Chapter 7 Reactions in Aqueous Solutions PowerPoint Presentation Which Ions Are Not Soluble In Water Solubility is a result of an. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. A saturated solution is one in. salts of some halides are generally soluble. this is a list of the solubility rules for ionic solids in water. some ions can be toxic when. Which Ions Are Not Soluble In Water.
From www.chegg.com
Solved Some solubility rules for ionic compounds in water Which Ions Are Not Soluble In Water substances that dissolve in water to yield ions are called electrolytes. some ions can be toxic when they separate in a solution but are helpful as part of a compound. A saturated solution is one in. The salts of silver (ag +),. this is a list of the solubility rules for ionic solids in water. Nonelectrolytes are. Which Ions Are Not Soluble In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID2276550 Which Ions Are Not Soluble In Water Solubility is a result of an. A saturated solution is one in. Nonelectrolytes are substances that do not produce ions. Solubility rules can be used to predict whether a large number of ionic. The salts of silver (ag +),. substances that dissolve in water to yield ions are called electrolytes. substances that dissolve in water to yield ions. Which Ions Are Not Soluble In Water.
From slideplayer.com
Chapter 4 Reactions in Aqueous Solution ppt download Which Ions Are Not Soluble In Water a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. salts of some halides are generally soluble. this is a list of the solubility rules for ionic solids in water. Solubility rules can be used to predict whether a large number of ionic. The salts of silver (ag +),.. Which Ions Are Not Soluble In Water.
From wcponline.com
The Basics of Ion Exchange and Water ChemistryPart II, The Water Which Ions Are Not Soluble In Water The salts of silver (ag +),. Solubility rules can be used to predict whether a large number of ionic. salts of some halides are generally soluble. A saturated solution is one in. some ions can be toxic when they separate in a solution but are helpful as part of a compound. substances that dissolve in water to. Which Ions Are Not Soluble In Water.
From www.slideshare.net
Chapter 8 Reactions in Aqueous Solution Which Ions Are Not Soluble In Water some ions can be toxic when they separate in a solution but are helpful as part of a compound. Solubility rules can be used to predict whether a large number of ionic. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. The salts of silver (ag +),. salts. Which Ions Are Not Soluble In Water.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Which Ions Are Not Soluble In Water not all ionic compounds are soluble in water. A saturated solution is one in. Solubility rules can be used to predict whether a large number of ionic. substances that dissolve in water to yield ions are called electrolytes. this is a list of the solubility rules for ionic solids in water. some ions can be toxic. Which Ions Are Not Soluble In Water.
From www.slideserve.com
PPT Qualitative Analysis Carry out procedures to identify ions in Which Ions Are Not Soluble In Water substances that dissolve in water to yield ions are called electrolytes. Solubility is a result of an. A saturated solution is one in. The salts of silver (ag +),. Nonelectrolytes are substances that do not produce ions. salts of some halides are generally soluble. this is a list of the solubility rules for ionic solids in water.. Which Ions Are Not Soluble In Water.
From ar.inspiredpencil.com
Solubility Rules Table Chemistry Which Ions Are Not Soluble In Water salts of some halides are generally soluble. Solubility is a result of an. this is a list of the solubility rules for ionic solids in water. The salts of silver (ag +),. A saturated solution is one in. substances that dissolve in water to yield ions are called electrolytes. not all ionic compounds are soluble in. Which Ions Are Not Soluble In Water.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Which Ions Are Not Soluble In Water Solubility is a result of an. substances that dissolve in water to yield ions are called electrolytes. some ions can be toxic when they separate in a solution but are helpful as part of a compound. The salts of silver (ag +),. substances that dissolve in water to yield ions are called electrolytes. a solubility chart. Which Ions Are Not Soluble In Water.
From www.numerade.com
SOLVED Solubility Rulesfor some ionic compounds in water Soluble Ionic Which Ions Are Not Soluble In Water Nonelectrolytes are substances that do not produce ions. Solubility is a result of an. substances that dissolve in water to yield ions are called electrolytes. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. not all ionic compounds are soluble in water. this is a list of. Which Ions Are Not Soluble In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Which Ions Are Not Soluble In Water a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. Nonelectrolytes are substances that do not produce ions. Solubility rules can be used to predict whether a large number of ionic. salts of some halides are generally soluble. The salts of silver (ag +),. this is a list of. Which Ions Are Not Soluble In Water.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is Which Ions Are Not Soluble In Water Solubility is a result of an. this is a list of the solubility rules for ionic solids in water. The salts of silver (ag +),. substances that dissolve in water to yield ions are called electrolytes. substances that dissolve in water to yield ions are called electrolytes. salts of some halides are generally soluble. some. Which Ions Are Not Soluble In Water.
From www.slideserve.com
PPT Solubility Rules and Precipitation Reactions PowerPoint Which Ions Are Not Soluble In Water Solubility is a result of an. A saturated solution is one in. substances that dissolve in water to yield ions are called electrolytes. this is a list of the solubility rules for ionic solids in water. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. some ions. Which Ions Are Not Soluble In Water.
From chem1180.blogspot.com
CHEM 1180 17.1 The Solubility Product Constant Which Ions Are Not Soluble In Water substances that dissolve in water to yield ions are called electrolytes. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. The salts of silver (ag +),. some ions can be toxic when they separate in a solution but are helpful as part of a compound. this is. Which Ions Are Not Soluble In Water.
From www.pathwaystochemistry.com
Precipitation Reactions Pathways to Chemistry Which Ions Are Not Soluble In Water Solubility rules can be used to predict whether a large number of ionic. Solubility is a result of an. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. The salts of silver (ag +),. substances that dissolve in water to yield ions are called electrolytes. a solubility. Which Ions Are Not Soluble In Water.
From www.chegg.com
Solved Some solubility rules for ionic compounds in water Which Ions Are Not Soluble In Water Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. some ions can be toxic when they separate in a solution but are helpful as part of a compound. Solubility is a result of an. a solubility chart is a chart describing whether the ionic compounds formed from. Which Ions Are Not Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Which Ions Are Not Soluble In Water Solubility is a result of an. salts of some halides are generally soluble. substances that dissolve in water to yield ions are called electrolytes. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. some ions can be toxic when they separate in a solution but are helpful. Which Ions Are Not Soluble In Water.
From exogctlqx.blob.core.windows.net
Examples Of Not Soluble In Water at Edward Brafford blog Which Ions Are Not Soluble In Water Solubility rules can be used to predict whether a large number of ionic. a solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and. salts of some halides are generally soluble. The salts of silver (ag +),. Nonelectrolytes are substances that do not produce ions. some ions can be toxic. Which Ions Are Not Soluble In Water.