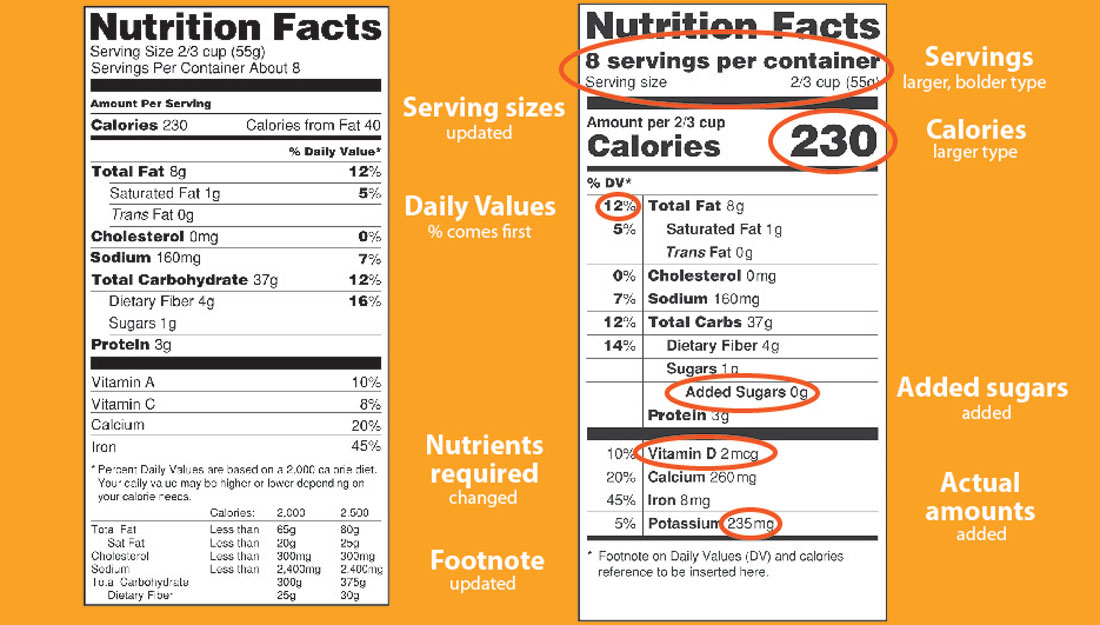
Fda Label Guidelines For Dietary Supplements . Fda has provided a guidance document. Nutrition labeling of dietary supplements. Nutrition labeling of raw fruit, vegetables, and fish. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. The dietary supplement health and education act of 1994 (the dshea) amended the act, in part, by defining dietary supplements, adding specific. The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as dietary. (a) (1) ingredients required to be declared on the label or labeling of a food,. You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. This review provides guidance for the content and format of labels,. § 101.36 nutrition labeling of dietary supplements. Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations.
from nutrition.ftempo.com
The dietary supplement health and education act of 1994 (the dshea) amended the act, in part, by defining dietary supplements, adding specific. Fda has provided a guidance document. Nutrition labeling of raw fruit, vegetables, and fish. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. This review provides guidance for the content and format of labels,. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as dietary. (a) (1) ingredients required to be declared on the label or labeling of a food,. § 101.36 nutrition labeling of dietary supplements. Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations.
Fda Daily Nutritional Requirements Chart Nutrition Ftempo
Fda Label Guidelines For Dietary Supplements (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. The dietary supplement health and education act of 1994 (the dshea) amended the act, in part, by defining dietary supplements, adding specific. Fda has provided a guidance document. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. (a) (1) ingredients required to be declared on the label or labeling of a food,. Nutrition labeling of raw fruit, vegetables, and fish. § 101.36 nutrition labeling of dietary supplements. You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. Nutrition labeling of dietary supplements. The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as dietary. This review provides guidance for the content and format of labels,. Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations.
From ar.inspiredpencil.com
Dietary Supplements Label Fda Label Guidelines For Dietary Supplements This review provides guidance for the content and format of labels,. Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations. § 101.36 nutrition labeling of dietary supplements. Nutrition labeling of dietary supplements. You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement.. Fda Label Guidelines For Dietary Supplements.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Fda Label Guidelines For Dietary Supplements Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. § 101.36 nutrition labeling of dietary supplements. (a) (1) ingredients required to be declared on the label or labeling of a food,. The dietary. Fda Label Guidelines For Dietary Supplements.
From www.crnusa.org
How to Read a Supplement Label Council for Responsible Nutrition Fda Label Guidelines For Dietary Supplements Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations. You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. § 101.36 nutrition labeling of dietary supplements. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling. Fda Label Guidelines For Dietary Supplements.
From esha.com
New FDA Nutrition Facts Label Font Style and Size ESHA Research Fda Label Guidelines For Dietary Supplements (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. Nutrition labeling of raw fruit, vegetables, and fish. This review provides guidance for the content and format of labels,. § 101.36 nutrition labeling of dietary supplements. The dietary supplement health and education act of 1994 (the dshea) amended the act, in part,. Fda Label Guidelines For Dietary Supplements.
From commons.wikimedia.org
FileFDA Nutrition Facts Label 2014.jpg Wikimedia Commons Fda Label Guidelines For Dietary Supplements You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. § 101.36 nutrition labeling of dietary supplements. Fda has provided a guidance document. The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as dietary. (a) (1) ingredients required to be declared. Fda Label Guidelines For Dietary Supplements.
From nutrition.ftempo.com
Fda Nutrition Label Guidelines 2017 Nutrition Ftempo Fda Label Guidelines For Dietary Supplements Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. This review provides guidance for the content and format of labels,. The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as dietary. (a) (1) ingredients required to be declared on the label or labeling. Fda Label Guidelines For Dietary Supplements.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Supplements Fda Label Guidelines For Dietary Supplements Nutrition labeling of raw fruit, vegetables, and fish. § 101.36 nutrition labeling of dietary supplements. Fda has provided a guidance document. Nutrition labeling of dietary supplements. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. This review provides guidance for the content and format of labels,. Package labels for foods and dietary supplements. Fda Label Guidelines For Dietary Supplements.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Fda Label Guidelines For Dietary Supplements The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as dietary. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. Fda has provided a guidance. Fda Label Guidelines For Dietary Supplements.
From vipcolor.com
Keeping up with the FDA food label regulations VIPColor Fda Label Guidelines For Dietary Supplements (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. The dietary supplement health and education act of 1994 (the dshea) amended the act, in part, by defining dietary supplements, adding specific. The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as. Fda Label Guidelines For Dietary Supplements.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Fda Label Guidelines For Dietary Supplements Nutrition labeling of dietary supplements. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. Fda has provided a guidance document. The dietary supplement label database (dsld) captures the image and all information declared on. Fda Label Guidelines For Dietary Supplements.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Fda Label Guidelines For Dietary Supplements (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations. § 101.36 nutrition labeling of. Fda Label Guidelines For Dietary Supplements.
From www.menusano.com
How to Create a FDA Approved Nutrition Fact Label MenuSano Fda Label Guidelines For Dietary Supplements The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as dietary. Nutrition labeling of dietary supplements. Nutrition labeling of raw fruit, vegetables, and fish. § 101.36 nutrition labeling of dietary supplements. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. (a) the label. Fda Label Guidelines For Dietary Supplements.
From www.fda.gov
Nutrition Facts Label Images for Download FDA Fda Label Guidelines For Dietary Supplements You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. Nutrition labeling of dietary supplements. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. Fda has. Fda Label Guidelines For Dietary Supplements.
From digitalcolorinc.com
FDA Nutrition Label Updates What You Need to Know Digital Color Inc. Fda Label Guidelines For Dietary Supplements You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. § 101.36 nutrition labeling of dietary supplements. This review provides guidance for the content and format of labels,. Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations. Fda has provided a guidance. Fda Label Guidelines For Dietary Supplements.
From outsource.contractlaboratory.com
New FDA Food Labeling Guidelines Include Nutrition Facts Requirements Fda Label Guidelines For Dietary Supplements Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. This review provides guidance for the content and format of labels,. Nutrition labeling of dietary supplements. (a) (1) ingredients required to be declared on the label. Fda Label Guidelines For Dietary Supplements.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Fda Label Guidelines For Dietary Supplements (a) (1) ingredients required to be declared on the label or labeling of a food,. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. Nutrition labeling of dietary supplements. This review provides guidance for the content and format of labels,. Package labels for foods and dietary supplements must conform with title. Fda Label Guidelines For Dietary Supplements.
From ollivierclimbs.blogspot.com
Fda Nutrition Label Guidelines 2020 Fda Label Guidelines For Dietary Supplements Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. The dietary supplement health and education act of 1994 (the dshea) amended the act, in part, by defining dietary supplements, adding specific. The dietary. Fda Label Guidelines For Dietary Supplements.
From www.statnews.com
FDA’s plan to define 'healthy' for food packaging Do we really need it? Fda Label Guidelines For Dietary Supplements The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as dietary. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. Nutrition labeling of raw fruit, vegetables, and fish. § 101.36 nutrition labeling of dietary supplements. (a) the label of a dietary supplement that. Fda Label Guidelines For Dietary Supplements.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Supplements Fda Label Guidelines For Dietary Supplements (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. This review provides guidance for the content and format of labels,. You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. Nutrition labeling of dietary supplements. The dietary supplement label database (dsld) captures. Fda Label Guidelines For Dietary Supplements.
From www.onlinelabels.com
What You Need to Know About the New FDA Nutrition Fact Label Fda Label Guidelines For Dietary Supplements You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations. The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as dietary. Nutrition labeling. Fda Label Guidelines For Dietary Supplements.
From cehrjpce.blob.core.windows.net
Fda Label Examples at Claude Patterson blog Fda Label Guidelines For Dietary Supplements The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as dietary. Nutrition labeling of dietary supplements. This review provides guidance for the content and format of labels,. The dietary supplement health and education act of 1994 (the dshea) amended the act, in part, by defining dietary supplements, adding specific. Fda. Fda Label Guidelines For Dietary Supplements.
From www.nfpt.com
Understanding the Changes to the 2018 FDA Nutrition Label Fda Label Guidelines For Dietary Supplements Nutrition labeling of dietary supplements. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. § 101.36 nutrition labeling of dietary supplements. The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as dietary. (a) (1) ingredients required to be declared on the label or. Fda Label Guidelines For Dietary Supplements.
From ubiquinol.org
How to Read a Supplement Label Fda Label Guidelines For Dietary Supplements Fda has provided a guidance document. § 101.36 nutrition labeling of dietary supplements. Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. Nutrition labeling of raw fruit, vegetables, and fish. (a) the label of a. Fda Label Guidelines For Dietary Supplements.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Fda Label Guidelines For Dietary Supplements The dietary supplement label database (dsld) captures the image and all information declared on the labels of products sold as dietary. This review provides guidance for the content and format of labels,. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. The dietary supplement health and education act of 1994 (the dshea) amended. Fda Label Guidelines For Dietary Supplements.
From astronovaproductid.com
How to Comply with FDA Requirements for Dietary Supplement Labeling Fda Label Guidelines For Dietary Supplements Nutrition labeling of raw fruit, vegetables, and fish. This review provides guidance for the content and format of labels,. Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations. (a) (1) ingredients required to be declared on the label or labeling of a food,. Fda has provided a guidance document. Nutrition labeling. Fda Label Guidelines For Dietary Supplements.
From foodlabelmaker.com
FDA Nutrition Facts Label Font Size & Style Guidelines Fda Label Guidelines For Dietary Supplements This review provides guidance for the content and format of labels,. (a) (1) ingredients required to be declared on the label or labeling of a food,. The dietary supplement health and education act of 1994 (the dshea) amended the act, in part, by defining dietary supplements, adding specific. Nutrition labeling of raw fruit, vegetables, and fish. § 101.36 nutrition labeling. Fda Label Guidelines For Dietary Supplements.
From www.rni-consulting.com
How to achieve compliance in the USA? Dietary supplement labels RNI Fda Label Guidelines For Dietary Supplements § 101.36 nutrition labeling of dietary supplements. The dietary supplement health and education act of 1994 (the dshea) amended the act, in part, by defining dietary supplements, adding specific. Nutrition labeling of raw fruit, vegetables, and fish. Fda has provided a guidance document. This review provides guidance for the content and format of labels,. The dietary supplement label database (dsld). Fda Label Guidelines For Dietary Supplements.
From www.fda.gov
Guidance for Industry Guide for Developing and Using Data Bases for Fda Label Guidelines For Dietary Supplements Fda has provided a guidance document. (a) (1) ingredients required to be declared on the label or labeling of a food,. Nutrition labeling of raw fruit, vegetables, and fish. You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. Package labels for foods and dietary supplements must conform with title 21. Fda Label Guidelines For Dietary Supplements.
From www.fodmapeveryday.com
How to Read a FDA Nutrition Facts Label FODMAP Everyday Fda Label Guidelines For Dietary Supplements (a) (1) ingredients required to be declared on the label or labeling of a food,. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. This review provides guidance for the content and format of labels,.. Fda Label Guidelines For Dietary Supplements.
From www.fda.gov
FDA In Brief FDA provides additional information about requirements Fda Label Guidelines For Dietary Supplements The dietary supplement health and education act of 1994 (the dshea) amended the act, in part, by defining dietary supplements, adding specific. Nutrition labeling of dietary supplements. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance. Fda has provided a guidance document. Nutrition labeling of raw fruit, vegetables, and fish. (a). Fda Label Guidelines For Dietary Supplements.
From lieflabs.com
Supplement Labels 101 Lief Labs Fda Label Guidelines For Dietary Supplements Nutrition labeling of raw fruit, vegetables, and fish. (a) (1) ingredients required to be declared on the label or labeling of a food,. Fda has provided a guidance document. Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations. Nutrition labeling of dietary supplements. The dietary supplement label database (dsld) captures the. Fda Label Guidelines For Dietary Supplements.
From cohenhealthcarelaw.com
FDA Dietary Supplement Labeling Requirements Comply or Die Cohen Fda Label Guidelines For Dietary Supplements Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations. Nutrition labeling of dietary supplements. Nutrition labeling of raw fruit, vegetables, and fish. You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. (a) the label of a dietary supplement that is offered. Fda Label Guidelines For Dietary Supplements.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Fda Label Guidelines For Dietary Supplements This review provides guidance for the content and format of labels,. § 101.36 nutrition labeling of dietary supplements. You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. Nutrition labeling of dietary supplements. Package labels for foods and dietary supplements must conform with title 21 of the code of federal regulations.. Fda Label Guidelines For Dietary Supplements.
From nutrition.ftempo.com
Fda Daily Nutritional Requirements Chart Nutrition Ftempo Fda Label Guidelines For Dietary Supplements (a) (1) ingredients required to be declared on the label or labeling of a food,. This review provides guidance for the content and format of labels,. You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement. Fda Label Guidelines For Dietary Supplements.
From foodindustryexecutive.com
FDA Final Guidance Clarifies New Nutrition Label Requirements Food Fda Label Guidelines For Dietary Supplements Questions and answers regarding the labeling of dietary supplements as required by the dietary supplement and. You must implement quality control operations in your manufacturing, packaging, labeling, and holding operations for producing the dietary supplement. The dietary supplement health and education act of 1994 (the dshea) amended the act, in part, by defining dietary supplements, adding specific. Nutrition labeling of. Fda Label Guidelines For Dietary Supplements.