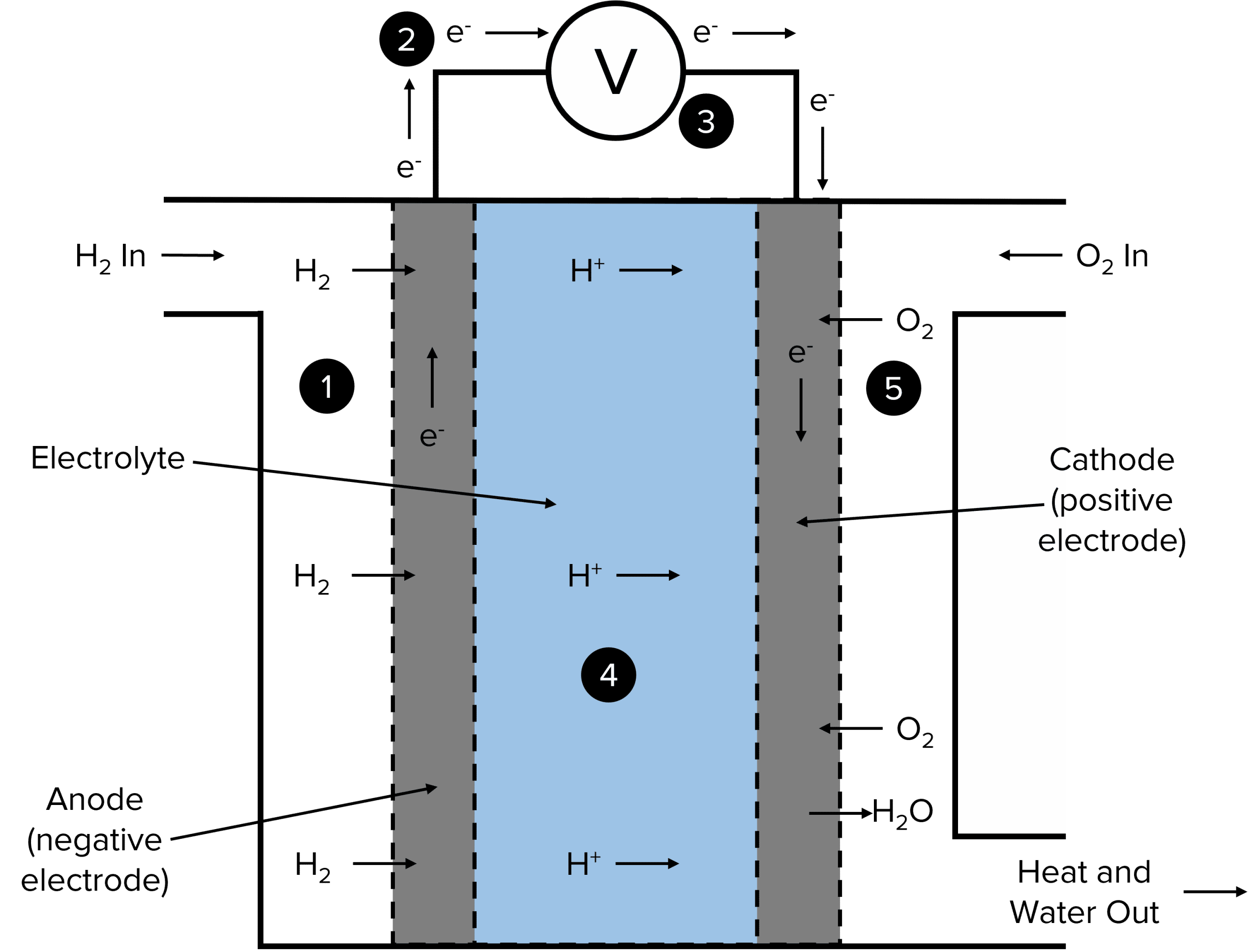
Examples Of Fuel Cell Class 12 . A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: In the presence of two electrodes, a. What is an example of a fuel cell? Learn the concepts of class 12 chemistry electrochemistry with videos and stories. Video lecture on fuel cell from electrochemistry chapter of chemistry class. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage over all.
from mmerevise.co.uk
Fuel cells have an important advantage over all. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: What is an example of a fuel cell? Video lecture on fuel cell from electrochemistry chapter of chemistry class. In the presence of two electrodes, a. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. Learn the concepts of class 12 chemistry electrochemistry with videos and stories.
Electrochemical Cells Worksheets and Revision MME
Examples Of Fuel Cell Class 12 In the presence of two electrodes, a. Learn the concepts of class 12 chemistry electrochemistry with videos and stories. Video lecture on fuel cell from electrochemistry chapter of chemistry class. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. Fuel cells have an important advantage over all. What is an example of a fuel cell? In the presence of two electrodes, a.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Examples Of Fuel Cell Class 12 A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. What is an example of a fuel cell? Video lecture on fuel cell from electrochemistry chapter of chemistry class. Fuel cells have an important advantage over all. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical. Examples Of Fuel Cell Class 12.
From www.pinterest.com
Working principle of fuel cell, parts of fuel cell in 2022 Fuel cell Examples Of Fuel Cell Class 12 A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. In the presence of two electrodes, a. What is an example of a fuel cell? Learn the concepts of class 12 chemistry electrochemistry with videos and stories. A fuel cell like this will continue to operate and produce electrical energy as long as. Examples Of Fuel Cell Class 12.
From www.youtube.com
L14 fuel cell class 12 h2o2 fuel cell nickel cadmium battery Examples Of Fuel Cell Class 12 What is an example of a fuel cell? A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Video lecture on fuel cell from electrochemistry chapter of chemistry class. Fuel cells have an important advantage over all. In the presence of two electrodes, a. Learn the. Examples Of Fuel Cell Class 12.
From www.youtube.com
Fuel Cell (class 12th chemistry ) YouTube Examples Of Fuel Cell Class 12 Video lecture on fuel cell from electrochemistry chapter of chemistry class. Fuel cells have an important advantage over all. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. Learn the concepts of class 12 chemistry electrochemistry with videos and stories. What is an example of a fuel cell? A fuel cell is. Examples Of Fuel Cell Class 12.
From manualdatawolf.z19.web.core.windows.net
Fuel Cell Circuit Diagram Examples Of Fuel Cell Class 12 A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. What is an example of a fuel cell? Learn the concepts of class 12 chemistry electrochemistry with videos and stories. A fuel cell is a tiny device, capable of generating electricity by force of a chemical. Examples Of Fuel Cell Class 12.
From www.slideserve.com
PPT Definition Types of Batteries Primary Batteries A. Lithium cell Examples Of Fuel Cell Class 12 What is an example of a fuel cell? A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. Fuel cells have an important advantage over all. Learn the concepts of class 12 chemistry electrochemistry with videos and stories. In the presence of two electrodes, a. Video lecture on fuel cell from electrochemistry chapter. Examples Of Fuel Cell Class 12.
From www.youtube.com
3.17Fuel cell class 12th,Electrochemistry YouTube Examples Of Fuel Cell Class 12 A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: Video lecture on fuel cell from electrochemistry chapter of chemistry class. Fuel cells have an important advantage over all. What is an example of a fuel cell? A fuel cell like this will continue to operate and produce electrical energy. Examples Of Fuel Cell Class 12.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Examples Of Fuel Cell Class 12 A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. In the presence of two electrodes, a. Learn the concepts of class 12 chemistry electrochemistry with videos and stories. What is an example of a fuel cell? A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical. Examples Of Fuel Cell Class 12.
From www.youtube.com
Trick for H2O2 Fuel cell Class 12th, Electrochemistry What are Examples Of Fuel Cell Class 12 What is an example of a fuel cell? Video lecture on fuel cell from electrochemistry chapter of chemistry class. Learn the concepts of class 12 chemistry electrochemistry with videos and stories. Fuel cells have an important advantage over all. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: A. Examples Of Fuel Cell Class 12.
From manualdiagramausterlitz.z19.web.core.windows.net
Fuel Cell Schematic Diagram Examples Of Fuel Cell Class 12 Learn the concepts of class 12 chemistry electrochemistry with videos and stories. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: Fuel cells have an important advantage over all. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. In the presence. Examples Of Fuel Cell Class 12.
From www.researchgate.net
Classification of fuel cells. Download Scientific Diagram Examples Of Fuel Cell Class 12 A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. Fuel cells have an important advantage over all. In the presence of two electrodes, a. A fuel cell is a tiny device, capable. Examples Of Fuel Cell Class 12.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Examples Of Fuel Cell Class 12 Fuel cells have an important advantage over all. Learn the concepts of class 12 chemistry electrochemistry with videos and stories. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. What is an example of a fuel cell? A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction,. Examples Of Fuel Cell Class 12.
From www.vedantu.com
What are ‘fuel cells’? Write cathode and anode reaction in a fuel cell. Examples Of Fuel Cell Class 12 Fuel cells have an important advantage over all. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. Learn the concepts of class 12 chemistry electrochemistry with videos and stories. What is an. Examples Of Fuel Cell Class 12.
From www.iswori.com.np
Electrochemistry Class 12 Chemistry Notes NEB Notes Examples Of Fuel Cell Class 12 Learn the concepts of class 12 chemistry electrochemistry with videos and stories. What is an example of a fuel cell? A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: In the presence of two electrodes, a. A fuel cell like this will continue to operate and produce electrical energy. Examples Of Fuel Cell Class 12.
From www.fuelcellstore.com
Fuel Cell Modeling Examples Of Fuel Cell Class 12 A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. Examples Of Fuel Cell Class 12.
From www.slideshare.net
Types of fuel cells Examples Of Fuel Cell Class 12 A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. Examples Of Fuel Cell Class 12.
From studymind.co.uk
Fuel Cells (GCSE Chemistry) Study Mind Examples Of Fuel Cell Class 12 Learn the concepts of class 12 chemistry electrochemistry with videos and stories. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Video lecture on fuel cell from electrochemistry chapter of chemistry class. A fuel cell is a tiny device, capable of generating electricity by force. Examples Of Fuel Cell Class 12.
From brainly.in
Define fuel cell and write its two advantages. Brainly.in Examples Of Fuel Cell Class 12 A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: Fuel cells have an important advantage over all. What is an example of a fuel cell? In the presence of two electrodes, a.. Examples Of Fuel Cell Class 12.
From www.youtube.com
Fuel Cells part 31 electro chemistry CBSE class 12 tricks By Examples Of Fuel Cell Class 12 A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: Video lecture on fuel cell from electrochemistry chapter of chemistry class. What is an example of a fuel cell? Learn the concepts of. Examples Of Fuel Cell Class 12.
From www.youtube.com
Trick for H2 O2 Fuel cells YouTube Examples Of Fuel Cell Class 12 A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. What is an example of a fuel cell? Learn the concepts of class 12 chemistry electrochemistry with videos and stories. In the presence of two electrodes, a. Fuel cells have an important advantage over all. A fuel cell can be described as an. Examples Of Fuel Cell Class 12.
From www.researchgate.net
Application of ALD on fuel cells. a) Different types of fuel cells with Examples Of Fuel Cell Class 12 What is an example of a fuel cell? Fuel cells have an important advantage over all. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. Learn the concepts of class 12 chemistry electrochemistry with videos and stories. A fuel cell like this will continue to operate and produce electrical energy as long. Examples Of Fuel Cell Class 12.
From www.youtube.com
fuel cells electrochemistry 2 class 12 chemistry subject notes lectures Examples Of Fuel Cell Class 12 A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. In the presence of two electrodes, a. What is an example of a fuel cell? Learn the concepts of class 12 chemistry electrochemistry with videos and stories. A fuel cell can be described as an electrochemical. Examples Of Fuel Cell Class 12.
From www.comsol.com
4 Examples of Fuel Cell Modeling in COMSOL Multiphysics® COMSOL Blog Examples Of Fuel Cell Class 12 Video lecture on fuel cell from electrochemistry chapter of chemistry class. Learn the concepts of class 12 chemistry electrochemistry with videos and stories. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. What is an example of a fuel cell? In the presence of two. Examples Of Fuel Cell Class 12.
From chemistnotes.com
Fuel cell Definition, types Chemistry Notes Examples Of Fuel Cell Class 12 Learn the concepts of class 12 chemistry electrochemistry with videos and stories. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. In the presence of two electrodes, a. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: Fuel cells have an. Examples Of Fuel Cell Class 12.
From www.youtube.com
FUEL CELLElectrochemistryChemistryClass 12 YouTube Examples Of Fuel Cell Class 12 What is an example of a fuel cell? A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. Fuel cells have an important advantage over all. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Learn the concepts. Examples Of Fuel Cell Class 12.
From www.youtube.com
Fuel cell Class 12 Chemistry Easy Explanation Video In English Or Examples Of Fuel Cell Class 12 Learn the concepts of class 12 chemistry electrochemistry with videos and stories. Video lecture on fuel cell from electrochemistry chapter of chemistry class. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell can be described as an electrochemical cell which, through an. Examples Of Fuel Cell Class 12.
From www.youtube.com
Electrochemistry( Part 6) Batteries, Fuel Cells, Corrosion NCERT Examples Of Fuel Cell Class 12 What is an example of a fuel cell? A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: Fuel cells have an important advantage over all. In the presence of two electrodes, a.. Examples Of Fuel Cell Class 12.
From www.geeksforgeeks.org
Fuel Cells Definition, Types, Advantages, Limitations Examples Of Fuel Cell Class 12 A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known. Examples Of Fuel Cell Class 12.
From www.engineeringa2z.com
Fuel Cell Construction and Working Engineeringa2z Examples Of Fuel Cell Class 12 Learn the concepts of class 12 chemistry electrochemistry with videos and stories. What is an example of a fuel cell? A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell like this will. Examples Of Fuel Cell Class 12.
From studiousguy.com
Fuel Cell Working Principle StudiousGuy Examples Of Fuel Cell Class 12 Learn the concepts of class 12 chemistry electrochemistry with videos and stories. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell like this will continue to operate and produce. Examples Of Fuel Cell Class 12.
From www.youtube.com
GCSE Chemistry Fuel Cells 45 YouTube Examples Of Fuel Cell Class 12 Fuel cells have an important advantage over all. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. Video lecture on fuel cell from electrochemistry chapter of chemistry class.. Examples Of Fuel Cell Class 12.
From www.chfca.ca
About Fuel Cells CHFCA Examples Of Fuel Cell Class 12 Video lecture on fuel cell from electrochemistry chapter of chemistry class. Fuel cells have an important advantage over all. What is an example of a fuel cell? A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. In the presence of two electrodes, a. A fuel. Examples Of Fuel Cell Class 12.
From www.youtube.com
What is a Fuel Cell?! GCSE Separate Chemistry 19 YouTube Examples Of Fuel Cell Class 12 What is an example of a fuel cell? Learn the concepts of class 12 chemistry electrochemistry with videos and stories. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. In the presence of two electrodes, a. Fuel cells have an important advantage over all. A fuel cell can be described as an. Examples Of Fuel Cell Class 12.
From thechemistrynotes.com
Fuel Cell Definition, Types, and Advantages Examples Of Fuel Cell Class 12 Video lecture on fuel cell from electrochemistry chapter of chemistry class. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. A device that converts energy of combustion of. Examples Of Fuel Cell Class 12.
From www.fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Examples Of Fuel Cell Class 12 Fuel cells have an important advantage over all. Video lecture on fuel cell from electrochemistry chapter of chemistry class. A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as: In the presence of two electrodes, a. Learn the concepts of class 12 chemistry electrochemistry with videos and stories. What is. Examples Of Fuel Cell Class 12.