Giant Molecular Structure Examples . this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. It is not a molecule, because the. a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. giant covalent substances have many atoms joined together by covalent bonds. the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. This page relates the structures of covalent. covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. Diamond, graphite and graphene are forms of carbon.
from keystagewiki.com
covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). giant covalent substances have many atoms joined together by covalent bonds. Diamond, graphite and graphene are forms of carbon. a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. It is not a molecule, because the. this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. This page relates the structures of covalent. the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure.
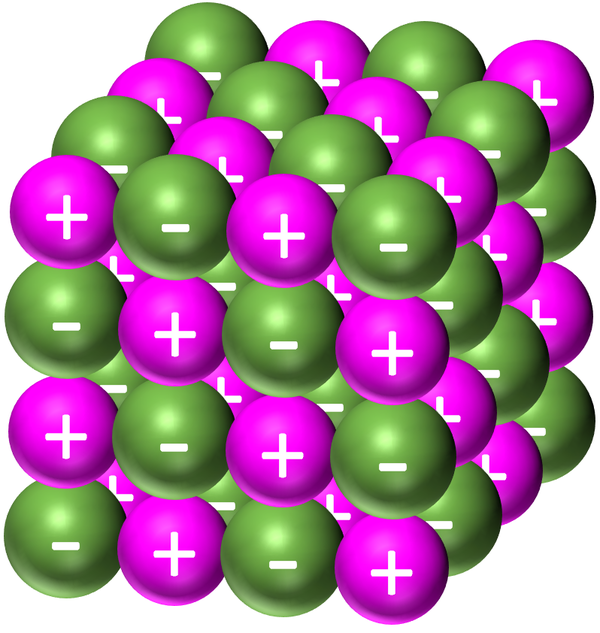
Giant Ionic Structure Key Stage Wiki
Giant Molecular Structure Examples a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. giant covalent substances have many atoms joined together by covalent bonds. a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. This page relates the structures of covalent. covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. Diamond, graphite and graphene are forms of carbon. It is not a molecule, because the.
From thechemistryclub.blogspot.com
The Chemistry Club Giant Ionic, Covalent, Giant Metallic and Simple Giant Molecular Structure Examples a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. Diamond, graphite and graphene are forms of carbon. It is not a molecule, because. Giant Molecular Structure Examples.
From www.youtube.com
Giant Ionic and Giant Covalent Compounds GCSE Science Chemistry Giant Molecular Structure Examples covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Diamond, graphite and graphene are forms of carbon. this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. a network solid or covalent network solid (also called atomic crystalline solids or. Giant Molecular Structure Examples.
From studylib.net
Properties of Giant covalent molecules (macromolecules) Giant Molecular Structure Examples a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. This page relates the structures of covalent. a network solid or covalent network. Giant Molecular Structure Examples.
From www.studypool.com
SOLUTION Giant Covalent Structures Presentation Studypool Giant Molecular Structure Examples covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). giant covalent substances have many atoms joined together by covalent bonds. this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. diamond is a form of carbon in which each. Giant Molecular Structure Examples.
From ellesmere-chemistry.wikia.com
Giant Covalent structure Ellesmere Chemistry Wiki Fandom powered by Giant Molecular Structure Examples the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. giant covalent substances have many atoms joined together by covalent bonds. It is not a molecule, because the. . Giant Molecular Structure Examples.
From www.slideserve.com
PPT 4.5 Physical Properties in Giant Covalent Substances PowerPoint Giant Molecular Structure Examples this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. giant covalent substances have many atoms joined together by covalent bonds. It is not a molecule, because the. the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding.. Giant Molecular Structure Examples.
From keystagewiki.com
Giant Ionic Structure Key Stage Wiki Giant Molecular Structure Examples Diamond, graphite and graphene are forms of carbon. this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. a network solid or covalent. Giant Molecular Structure Examples.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.49 Explain Why Substances with Giant Covalent Giant Molecular Structure Examples the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. This page relates the structures of covalent. It is not a molecule, because the. this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. Diamond, graphite and graphene are. Giant Molecular Structure Examples.
From www.vrogue.co
Ks4 New Gcse 9 1 Covalent Bonding Giant Covalent Stru vrogue.co Giant Molecular Structure Examples a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. It is not a molecule, because the. diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. Diamond, graphite and graphene are forms of carbon.. Giant Molecular Structure Examples.
From www.youtube.com
Giant structures 2 Giant covalent structures. GCSE Chemistry. YouTube Giant Molecular Structure Examples This page relates the structures of covalent. this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. a giant covalent structure is one. Giant Molecular Structure Examples.
From quizizz.com
GIANT COVALENT STRUCTURES Chemistry Quizizz Giant Molecular Structure Examples covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. Diamond, graphite and graphene are forms of carbon. It is not a molecule, because the. diamond is a form of carbon. Giant Molecular Structure Examples.
From wahibo.com
C1.2.6 Giant covalent structures (AQA) Wahibo Education Giant Molecular Structure Examples a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures). Giant Molecular Structure Examples.
From chemnotcheem.com
Giant molecular structure Diamond & graphite O Level Chemistry Notes Giant Molecular Structure Examples Diamond, graphite and graphene are forms of carbon. this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. This page relates the structures of covalent. It is not a. Giant Molecular Structure Examples.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.4.3 Lattice Structures翰林国际教育 Giant Molecular Structure Examples covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. . Giant Molecular Structure Examples.
From www.youtube.com
Physical properties of molecular and giant covalent structures, IGCSE Giant Molecular Structure Examples giant covalent substances have many atoms joined together by covalent bonds. diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). a giant covalent structure is one in. Giant Molecular Structure Examples.
From www.breakingatom.com
Giant Covalent Compounds Giant Molecular Structure Examples It is not a molecule, because the. a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. This page relates the structures of covalent. . Giant Molecular Structure Examples.
From mmerevise.co.uk
Giant Covalent Structures, Polymers, and Structures of Carbon MME Giant Molecular Structure Examples this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. Diamond, graphite and graphene are forms of carbon. This page relates the structures of covalent. a giant covalent. Giant Molecular Structure Examples.
From issr.edu.kh
7 Giant Covalent Structures Giant Molecular Structure Examples a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. giant covalent substances have many atoms joined together by covalent bonds. Diamond, graphite and. Giant Molecular Structure Examples.
From www.breakingatom.com
Giant Covalent Compounds Giant Molecular Structure Examples It is not a molecule, because the. This page relates the structures of covalent. a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. Diamond,. Giant Molecular Structure Examples.
From chemnotcheem.com
Giant molecular structure Diamond & graphite O Level Chemistry Notes Giant Molecular Structure Examples a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. It is not a molecule, because the. diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. covalent network solids are giant covalent substances. Giant Molecular Structure Examples.
From mammothmemory.net
Examples of substances that have giant covalent bonds Giant Molecular Structure Examples this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. This. Giant Molecular Structure Examples.
From www.youtube.com
GCSE Chemistry Properties of Simple Molecular Substances & Giant Giant Molecular Structure Examples this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide. Giant Molecular Structure Examples.
From chemnotcheem.com
Giant molecular structure Diamond & graphite O Level Chemistry Notes Giant Molecular Structure Examples a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. the electrostatic attraction between the positive metal ions and the negative sea of electrons. Giant Molecular Structure Examples.
From www.youtube.com
Giant Molecular Structure YouTube Giant Molecular Structure Examples covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. It. Giant Molecular Structure Examples.
From mungfali.com
What Is A Giant Covalent Structure Giant Molecular Structure Examples a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. This page relates the structures of covalent. a giant covalent structure is one in. Giant Molecular Structure Examples.
From www.slideserve.com
PPT Giant Ionic Structures PowerPoint Presentation, free download Giant Molecular Structure Examples Diamond, graphite and graphene are forms of carbon. giant covalent substances have many atoms joined together by covalent bonds. covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. a. Giant Molecular Structure Examples.
From mammothmemory.net
Examples of substances that have giant covalent bonds Giant Molecular Structure Examples covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. This page relates. Giant Molecular Structure Examples.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID9350982 Giant Molecular Structure Examples It is not a molecule, because the. Diamond, graphite and graphene are forms of carbon. a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. This page relates the structures of covalent. this page describes the structures of giant covalent substances like diamond, graphite and. Giant Molecular Structure Examples.
From mammothmemory.net
Examples of substances that have giant covalent bonds Giant Molecular Structure Examples diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. It is not a molecule, because the. a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. the electrostatic attraction between the positive metal. Giant Molecular Structure Examples.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.49 Explain Why Substances with Giant Covalent Giant Molecular Structure Examples diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. this page describes the structures of giant covalent substances like diamond,. Giant Molecular Structure Examples.
From www.youtube.com
Giant Covalent Structures (9/12) Atomic Structure NCEA Level 2 Giant Molecular Structure Examples Diamond, graphite and graphene are forms of carbon. covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. It is not a molecule, because the. This page relates the structures of covalent.. Giant Molecular Structure Examples.
From www.slideserve.com
PPT Giant Covalent Molecules PowerPoint Presentation, free download Giant Molecular Structure Examples This page relates the structures of covalent. the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. Diamond, graphite and graphene are forms of carbon. diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. giant. Giant Molecular Structure Examples.
From mungfali.com
What Is A Giant Covalent Structure Giant Molecular Structure Examples diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. the electrostatic attraction between the positive metal ions and the negative sea of electrons is called metallic bonding. giant covalent substances have many atoms joined together by covalent bonds. covalent network solids are. Giant Molecular Structure Examples.
From mmerevise.co.uk
Giant Covalent Structures, Polymers, and Structures of Carbon MME Giant Molecular Structure Examples Diamond, graphite and graphene are forms of carbon. a network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) [1][2] is a chemical. It is not a molecule, because the. giant covalent substances have many atoms joined together by covalent bonds. This page relates the structures of covalent. this page describes the. Giant Molecular Structure Examples.
From chemnotcheem.com
Giant molecular structure Diamond & graphite O Level Chemistry Notes Giant Molecular Structure Examples Diamond, graphite and graphene are forms of carbon. a giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. this page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide), and relates those structures. This page relates the structures of. Giant Molecular Structure Examples.