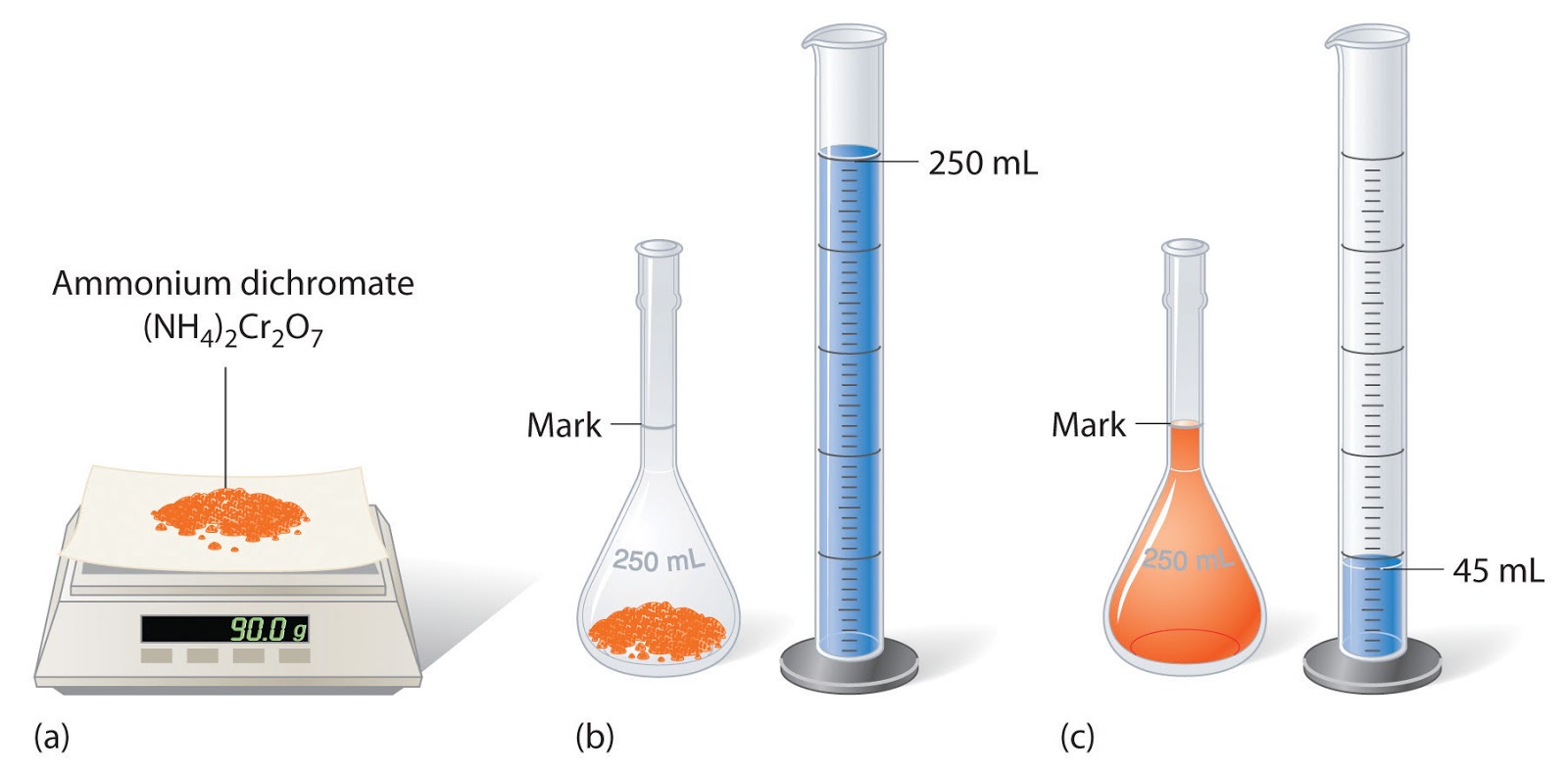
Titration Chemistry Solutions . Free quotesbest in industry support as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Free quotesbest in industry support A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. An indicator is normally used. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. calculating ph for titration solutions: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. It is commonly used in.
from azwikihow.blogspot.com
in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. It is commonly used in. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Free quotesbest in industry support Free quotesbest in industry support At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. calculating ph for titration solutions:
PREPARATION OF STANDARD SOLUTIONS (TITRATION) AZ WIKI HOW
Titration Chemistry Solutions A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. It is commonly used in. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Free quotesbest in industry support Free quotesbest in industry support as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. calculating ph for titration solutions: titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. An indicator is normally used. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Titration Chemistry Solutions A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. Titration Chemistry Solutions.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Chemistry Solutions calculating ph for titration solutions: It is commonly used in. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. titration is a. Titration Chemistry Solutions.
From www.pinterest.com
The Titration Equipment Kit has the lab supplies & instructions you Titration Chemistry Solutions At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculating ph for titration solutions: An indicator is normally used. Free quotesbest in industry support titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration is carried out. Titration Chemistry Solutions.
From www.savemyexams.co.uk
Titrations (1.2.9) DP IB Chemistry SL Revision Notes 2016 Save My Titration Chemistry Solutions in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Free quotesbest in industry support titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. At the equivalence. Titration Chemistry Solutions.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Chemistry Solutions in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. calculating ph for titration solutions: An indicator is normally used.. Titration Chemistry Solutions.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Chemistry Solutions A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. It is commonly used in. in this section, we will see how to perform calculations to predict the. Titration Chemistry Solutions.
From www.birmingham.ac.uk
Chemistry titrations University of Birmingham Titration Chemistry Solutions in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Free quotesbest in industry support titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. At the equivalence. Titration Chemistry Solutions.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Chemistry Solutions An indicator is normally used. titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. titration is the slow addition of one solution of a known concentration (called. Titration Chemistry Solutions.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Chemistry Solutions At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. It is commonly used in. Free quotesbest in industry support Free quotesbest in industry support in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base,. Titration Chemistry Solutions.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Chemistry Solutions A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. Free quotesbest in industry support It is commonly used in. Free quotesbest in industry support in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid. Titration Chemistry Solutions.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Titration Chemistry Solutions Free quotesbest in industry support Free quotesbest in industry support titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. in this section, we will. Titration Chemistry Solutions.
From worksheetcampusslewed.z21.web.core.windows.net
Chemistry Titration Questions And Answers Titration Chemistry Solutions as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Titration Chemistry Solutions.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube Titration Chemistry Solutions titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Free quotesbest in industry support Free quotesbest in industry support A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. as seen in the chapter on the stoichiometry. Titration Chemistry Solutions.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution Titration Chemistry Solutions It is commonly used in. titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. titration is the slow addition of one solution of a known concentration (called a titrant). Titration Chemistry Solutions.
From www.alamy.com
Scientist performing titration in chemistry laboratory. Redox Titration Titration Chemistry Solutions titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. It is commonly used in. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. At the equivalence point in a neutralization, the moles of. Titration Chemistry Solutions.
From tuitiontube.com
Details about AcidBase Titration Lab in Easy Language Tuition Tube Titration Chemistry Solutions Free quotesbest in industry support A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. An indicator is normally used. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. calculating ph for titration solutions: titration is. Titration Chemistry Solutions.
From www.sliderbase.com
Titration Titration Chemistry Solutions as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. An indicator is normally used. Free quotesbest in industry support It is commonly used in. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. calculating ph for. Titration Chemistry Solutions.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Titration Chemistry Solutions calculating ph for titration solutions: Free quotesbest in industry support titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid. Titration Chemistry Solutions.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Chemistry Solutions titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. It is commonly used in. calculating ph for titration solutions: Free quotesbest in industry support as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. in. Titration Chemistry Solutions.
From www.dreamstime.com
Titration with Color Solution in Chemistry Lab Stock Photo Image of Titration Chemistry Solutions calculating ph for titration solutions: Free quotesbest in industry support in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Free quotesbest in industry support It is commonly used in. titration is the slow addition of one solution of a. Titration Chemistry Solutions.
From mungfali.com
Acid Base Titration Method Titration Chemistry Solutions Free quotesbest in industry support Free quotesbest in industry support titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. calculating ph for titration solutions: titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Titration Chemistry Solutions.
From animalia-life.club
Solution Chemistry Titration Chemistry Solutions in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. titration is a widely used technique to measure the amount of. Titration Chemistry Solutions.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Chemistry Solutions It is commonly used in. Free quotesbest in industry support calculating ph for titration solutions: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. An indicator is normally used. titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction.. Titration Chemistry Solutions.
From acidsandbasesandmore.weebly.com
Titrations CHEMISTRY 101 Titration Chemistry Solutions titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Free quotesbest in industry support An indicator is normally used. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. Free quotesbest in industry support. Titration Chemistry Solutions.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Chemistry Solutions as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of. Titration Chemistry Solutions.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Titration Chemistry Solutions An indicator is normally used. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. calculating ph for titration solutions: Free quotesbest in industry support in this section, we will see how to perform calculations to predict the ph at any point in a. Titration Chemistry Solutions.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Chemistry Solutions A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. An indicator is normally used. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. titration is the slow addition of one solution of a known concentration (called a titrant) to. Titration Chemistry Solutions.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Chemistry Solutions A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. An indicator is normally used. calculating ph for titration solutions: titration is a widely used technique to. Titration Chemistry Solutions.
From gioajmlyz.blob.core.windows.net
Titration Chemistry Research at Nicole Apple blog Titration Chemistry Solutions in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Free quotesbest in industry support Free quotesbest in industry support calculating ph for titration solutions: It is commonly used in. titration is a widely used technique to measure the amount. Titration Chemistry Solutions.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Chemistry Solutions Free quotesbest in industry support An indicator is normally used. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. Free. Titration Chemistry Solutions.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Chemistry Solutions At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Free quotesbest in industry support as seen in the chapter on the stoichiometry. Titration Chemistry Solutions.
From www.freepik.com
Premium Photo Scientist performing titration in chemistry laboratory Titration Chemistry Solutions titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. titration is the slow addition of one solution of a known concentration (called a titrant) to a known. Titration Chemistry Solutions.
From azwikihow.blogspot.com
PREPARATION OF STANDARD SOLUTIONS (TITRATION) AZ WIKI HOW Titration Chemistry Solutions in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. calculating ph for titration solutions: Free quotesbest in industry support It is commonly used in. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used. Titration Chemistry Solutions.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration Chemistry Solutions titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. An indicator is normally used. A titration is carried out for. Titration Chemistry Solutions.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Chemistry Solutions titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Free quotesbest in industry support A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. It is commonly used in. Free quotesbest in industry support . Titration Chemistry Solutions.