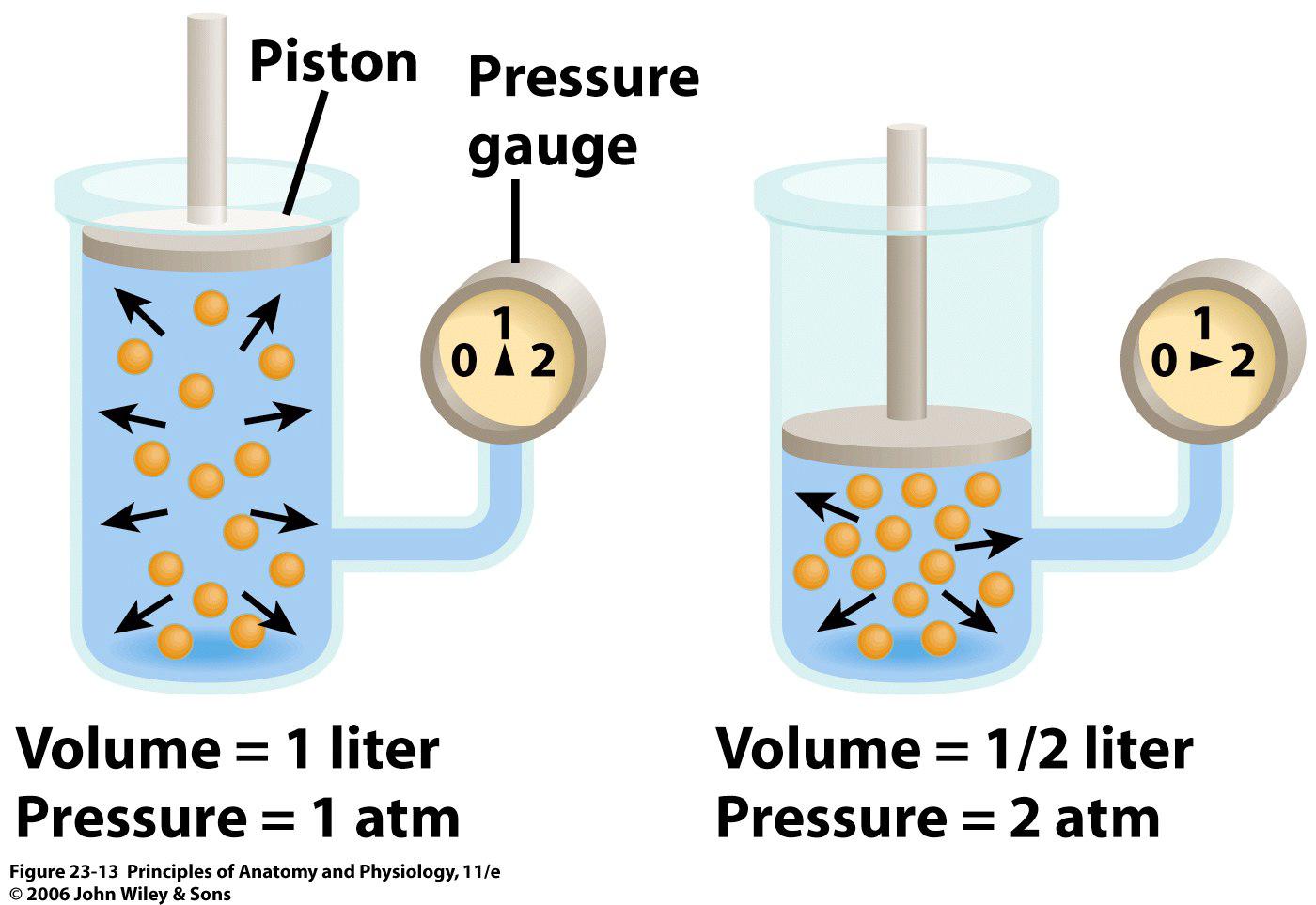
Boyle's Experiment Pressure . A gas (air) was trapped in the sealed end of the tube and. Pv = constant this means the pressure must be calculated from the. I.e., in equation form, pv = k, a constant. Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Boyle’s law can be represented by the equation: This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; At constant temperature, the volume of a fixed amount.
from manualworshipped.z14.web.core.windows.net
Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. A gas (air) was trapped in the sealed end of the tube and. Boyle’s law can be represented by the equation: At constant temperature, the volume of a fixed amount. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: I.e., in equation form, pv = k, a constant. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Pv = constant this means the pressure must be calculated from the.
Concept Of Boyle's Law
Boyle's Experiment Pressure This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Pv = constant this means the pressure must be calculated from the. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: At constant temperature, the volume of a fixed amount. Boyle’s law can be represented by the equation: A gas (air) was trapped in the sealed end of the tube and. I.e., in equation form, pv = k, a constant.
From chem.libretexts.org
5.3 The Simple Gas Laws Boyle’s Law, Charles’s Law and Avogadro’s Law Boyle's Experiment Pressure This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Boyle’s law can be represented by the equation: Pv = constant this means the pressure must be calculated from the. I.e., in equation form, pv = k, a constant.. Boyle's Experiment Pressure.
From www.dreamstime.com
Boyle S Law Showing that Pressure and Volume Inversely Related in a Gas Boyle's Experiment Pressure Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: I.e., in equation form, pv = k,. Boyle's Experiment Pressure.
From www.sciencephoto.com
Boyle's law apparatus, illustration Stock Image C050/7502 Science Boyle's Experiment Pressure I.e., in equation form, pv = k, a constant. Pv = constant this means the pressure must be calculated from the. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: At constant temperature, the volume of a fixed amount. Boyle’s law can be represented by the equation: Boyle’s law,. Boyle's Experiment Pressure.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock Boyle's Experiment Pressure This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Boyle’s law can be represented by the equation: I.e., in equation form, pv = k, a constant. At constant temperature, the volume of a fixed amount. Pv = constant this means the pressure must be calculated from the. Boyle's law. Boyle's Experiment Pressure.
From www.britannica.com
Boyle’s law Definition, Equation, & Facts Britannica Boyle's Experiment Pressure Pv = constant this means the pressure must be calculated from the. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: I.e., in equation form, pv = k, a constant. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. At constant temperature, the. Boyle's Experiment Pressure.
From youtube.com
CTSC practical experiment Boyle's Law YouTube Boyle's Experiment Pressure This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. A gas (air) was trapped in the sealed end of the tube and. Boyle's. Boyle's Experiment Pressure.
From testbook.com
Study Variation in Volume with Pressure at Constant Temperature Boyle Boyle's Experiment Pressure I.e., in equation form, pv = k, a constant. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature;. Boyle's Experiment Pressure.
From www.alamy.com
Boyle's law apparatus for investigating the relationship beween the Boyle's Experiment Pressure Boyle’s law can be represented by the equation: Pv = constant this means the pressure must be calculated from the. A gas (air) was trapped in the sealed end of the tube and. I.e., in equation form, pv = k, a constant. At constant temperature, the volume of a fixed amount. Boyle’s law, a relation concerning the compression and expansion. Boyle's Experiment Pressure.
From manualworshipped.z14.web.core.windows.net
Boyle's Law Explained Simply Boyle's Experiment Pressure I.e., in equation form, pv = k, a constant. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Pv = constant this means. Boyle's Experiment Pressure.
From www.sliderbase.com
Boyle's Law Presentation Chemistry Boyle's Experiment Pressure This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Boyle’s law can be represented by the equation: Boyle’s. Boyle's Experiment Pressure.
From chemistryskills.com
Boyle’s Law Chemistry Skills Boyle's Experiment Pressure Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. I.e., in equation form, pv = k, a constant. Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated. Boyle's Experiment Pressure.
From www.sciencephoto.com
Boyle conducting pressure experiment, 1669 Stock Image C052/7496 Boyle's Experiment Pressure This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas. Boyle's Experiment Pressure.
From www.expii.com
Boyle's Law — Overview & Formula Expii Boyle's Experiment Pressure Boyle’s law can be represented by the equation: Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. A gas (air) was trapped in the sealed end of the tube and. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by the. Boyle's Experiment Pressure.
From www.doubtnut.com
In Boyles experiment for a given gas at different temperature the grap Boyle's Experiment Pressure Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: A gas (air) was trapped in the sealed end of the tube and. Pv = constant this means the pressure must be. Boyle's Experiment Pressure.
From www.alamy.com
Boyle's Law , Relationship between pressure and gas volume Stock Vector Boyle's Experiment Pressure At constant temperature, the volume of a fixed amount. A gas (air) was trapped in the sealed end of the tube and. I.e., in equation form, pv = k, a constant. Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. Boyle’s law, a relation concerning the compression and expansion of a. Boyle's Experiment Pressure.
From edulab.com
Boyle's Law Apparatus Edulab Boyle's Experiment Pressure A gas (air) was trapped in the sealed end of the tube and. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Boyle’s law can be represented by the equation: Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. At constant temperature, the volume of. Boyle's Experiment Pressure.
From www.sciencephoto.com
Boyle pressure experiment, artwork Stock Image C006/9744 Science Boyle's Experiment Pressure Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Pv = constant this means the pressure must be calculated from the. A gas (air) was trapped in the sealed end of. Boyle's Experiment Pressure.
From www.flinnsci.com
Boyle’s Law in a Bottle Pressure versus Volume—ChemTopic™ Lab Activity Boyle's Experiment Pressure Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. A gas (air) was trapped in the sealed end of the tube and. Boyle’s law can be represented by the equation: At constant temperature, the volume of a fixed amount. This empirical relation, formulated by the physicist robert boyle in 1662, states. Boyle's Experiment Pressure.
From chemistryteory.blogspot.com
chemistry Gas Laws Boyle’s Law Boyle's Experiment Pressure Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; This relationship between pressure and volume is known as boyle’s law, after its discoverer,. Boyle's Experiment Pressure.
From www.youtube.com
Boyle's law Explanation, Limitations and Applications Explained Boyle's Experiment Pressure Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Boyle’s law, a relation concerning the compression and expansion of a. Boyle's Experiment Pressure.
From www.studocu.com
Boyle's Law Summary Chemistry in Context Applying Chemistry to Boyle's Experiment Pressure I.e., in equation form, pv = k, a constant. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Boyle’s law can be represented by the equation: At constant temperature, the volume of a fixed amount. A gas (air). Boyle's Experiment Pressure.
From lavernemabel.blogspot.com
Boyle's Law Experiment Report Lab Boyles Law Gases Pressure X Boyle's Experiment Pressure I.e., in equation form, pv = k, a constant. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Pv = constant this means the pressure must be calculated from the. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely. Boyle's Experiment Pressure.
From galileo.phys.virginia.edu
Boyle Boyle's Experiment Pressure At constant temperature, the volume of a fixed amount. Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; I.e., in. Boyle's Experiment Pressure.
From schematiclistbenz77.z13.web.core.windows.net
What Is Boyle's Law Simple Boyle's Experiment Pressure A gas (air) was trapped in the sealed end of the tube and. I.e., in equation form, pv = k, a constant. Pv = constant this means the pressure must be calculated from the. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: This empirical relation, formulated by the. Boyle's Experiment Pressure.
From studiousguy.com
8 Boyle’s Law Examples in Real Life StudiousGuy Boyle's Experiment Pressure This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: At constant temperature, the volume of a fixed amount. Boyle’s law can be represented by the equation: This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies. Boyle's Experiment Pressure.
From www.grc.nasa.gov
Boyle's Law Boyle's Experiment Pressure A gas (air) was trapped in the sealed end of the tube and. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Boyle’s law can be represented by the equation: I.e., in equation form, pv = k, a constant. Pv = constant this means the pressure must be calculated. Boyle's Experiment Pressure.
From sciencenotes.org
Boyle's Law Definition, Formula, Example Boyle's Experiment Pressure I.e., in equation form, pv = k, a constant. At constant temperature, the volume of a fixed amount. Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. Pv = constant this means the pressure must be calculated from the. Boyle’s law, a relation concerning the compression and expansion of a gas. Boyle's Experiment Pressure.
From www.studocu.com
Boyle's Law experiment guide Boyle’s Law PressureVolume Boyle's Experiment Pressure At constant temperature, the volume of a fixed amount. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: I.e., in equation form, pv = k, a constant. Boyle's law is a gas law that. Boyle's Experiment Pressure.
From www.slideserve.com
PPT Graphing Boyle’s Law PowerPoint Presentation, free download ID Boyle's Experiment Pressure I.e., in equation form, pv = k, a constant. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Boyle’s law can be represented. Boyle's Experiment Pressure.
From www.visionlearning.com
Propiedades de Gases Chemistry Visionlearning Boyle's Experiment Pressure Pv = constant this means the pressure must be calculated from the. At constant temperature, the volume of a fixed amount. Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by the. Boyle's Experiment Pressure.
From www.thoughtco.com
What Is The Formula For Boyle's Law? Boyle's Experiment Pressure Boyle’s law can be represented by the equation: A gas (air) was trapped in the sealed end of the tube and. Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. At constant temperature, the volume of a fixed amount. This empirical relation, formulated by the physicist robert boyle in 1662, states. Boyle's Experiment Pressure.
From manualworshipped.z14.web.core.windows.net
Concept Of Boyle's Law Boyle's Experiment Pressure A gas (air) was trapped in the sealed end of the tube and. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Boyle's law is a gas law that describes the relationship between pressure and volume at a. Boyle's Experiment Pressure.
From www.pinterest.com
Discover Boyle's Law Pressure and Volume Relationship Boyle's Experiment Pressure Boyle's law is a gas law that describes the relationship between pressure and volume at a constant temperature. A gas (air) was trapped in the sealed end of the tube and. At constant temperature, the volume of a fixed amount. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows:. Boyle's Experiment Pressure.
From www.slideserve.com
PPT Chapter 5 Gases PowerPoint Presentation, free download ID593425 Boyle's Experiment Pressure Pv = constant this means the pressure must be calculated from the. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v). Boyle's Experiment Pressure.
From www.vernier.com
Boyle's Law PressureVolume Relationship in Gases > Experiment 6 from Boyle's Experiment Pressure Boyle’s law can be represented by the equation: This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: At constant temperature, the volume of a fixed amount. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies. Boyle's Experiment Pressure.