Lead With Water Reaction . The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. Throughout history, lead has been used to make water and sewer pipes; Type metal and other alloys; All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. However, when lead comes in contact with moist air reactivity with water increases. The layer is most likely lead hydroxy carbonate [8]. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Under normal conditions lead does not react with water. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Wrappings for food, tobacco, and other products;.
from www.cleanwater.org
Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Type metal and other alloys; All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Wrappings for food, tobacco, and other products;. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. Throughout history, lead has been used to make water and sewer pipes; Under normal conditions lead does not react with water. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. The layer is most likely lead hydroxy carbonate [8]. However, when lead comes in contact with moist air reactivity with water increases.
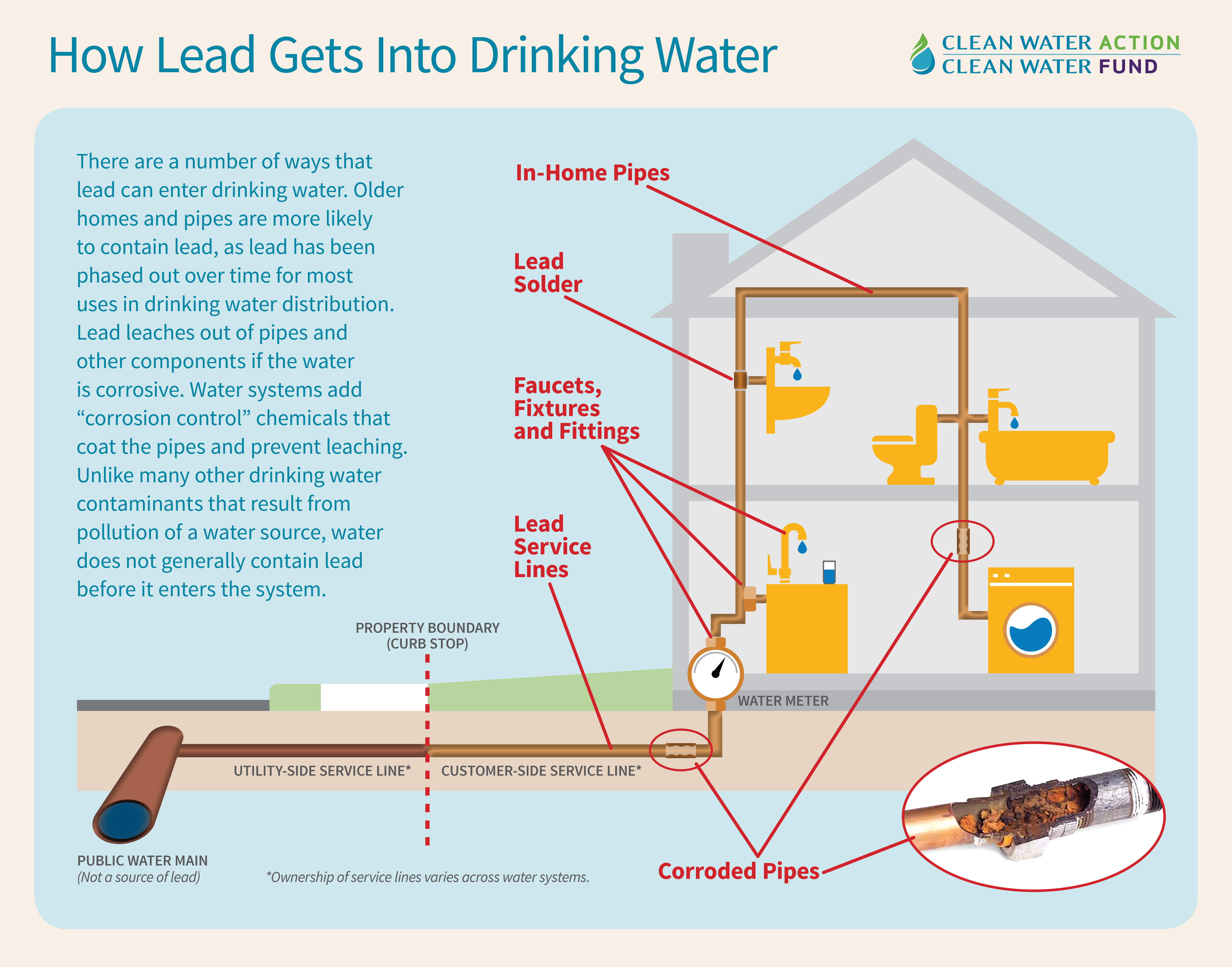
Lead and Drinking Water Clean Water Action
Lead With Water Reaction Type metal and other alloys; Under normal conditions lead does not react with water. The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Type metal and other alloys; Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. The layer is most likely lead hydroxy carbonate [8]. Wrappings for food, tobacco, and other products;. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. However, when lead comes in contact with moist air reactivity with water increases. Throughout history, lead has been used to make water and sewer pipes;
From www.youtube.com
Lead in Drinking Water YouTube Lead With Water Reaction Wrappings for food, tobacco, and other products;. Throughout history, lead has been used to make water and sewer pipes; The layer is most likely lead hydroxy carbonate [8]. Type metal and other alloys; Under normal conditions lead does not react with water. The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium,. Lead With Water Reaction.
From www.gleassociates.com
Here's What You Need to Know About Lead in Water Lead With Water Reaction Under normal conditions lead does not react with water. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. However, when lead comes in contact with moist air reactivity with water increases. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Type metal and. Lead With Water Reaction.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Lead With Water Reaction Under normal conditions lead does not react with water. The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: However, when lead comes in contact with moist air reactivity with water increases. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. Lead With Water Reaction.
From www.teachoo.com
In the double displacement reaction b/w aqueo (MCQ) Class 10 Science Lead With Water Reaction All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. The layer is most likely lead hydroxy carbonate [8]. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Under normal conditions lead does not react with water. The general reaction of calcium, strontium, and. Lead With Water Reaction.
From www.usatoday.com
By the numbers Lead contamination in drinking water Lead With Water Reaction Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. Throughout history, lead has been used to make water and sewer pipes; The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Wrappings for food, tobacco, and other products;. The layer is. Lead With Water Reaction.
From www.raritanheadwaters.org
Lead in Drinking Water Raritan Headwaters Lead With Water Reaction Throughout history, lead has been used to make water and sewer pipes; All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Type metal and other alloys; Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv). Lead With Water Reaction.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Lead With Water Reaction Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. The layer is most likely lead hydroxy carbonate [8]. Throughout history, lead has been used to make water and sewer pipes; However, when lead comes in contact with moist air reactivity with water increases. Under normal conditions lead does not react with. Lead With Water Reaction.
From www.premierh2o.com
Causes and Effects of Lead in Drinking Water Lead With Water Reaction All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. The layer is most likely lead hydroxy carbonate [8]. Type metal and other alloys; Throughout history, lead has been used to make water and sewer pipes; Under normal conditions lead does not react with water. Lead reacts with air, in the. Lead With Water Reaction.
From www.pvwc.com
Lead in Water How PVWC Lead With Water Reaction Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Throughout history, lead has been used to make water and sewer pipes; All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\),. Lead With Water Reaction.
From www.epa.gov
Basic Information about Lead in Drinking Water US EPA Lead With Water Reaction Under normal conditions lead does not react with water. The layer is most likely lead hydroxy carbonate [8]. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Wrappings for food, tobacco, and other products;. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. All. Lead With Water Reaction.
From www.alkaviva.com.au
Lead in Your Water? Lead With Water Reaction Wrappings for food, tobacco, and other products;. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Under normal conditions lead does not react with water. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Type metal and other alloys; However, when lead comes. Lead With Water Reaction.
From sites.google.com
Precipitation Reaction Science Lead With Water Reaction The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: The layer is most likely lead hydroxy carbonate [8]. Lead reacts with air, in the presence of moist and carbon dioxide,. Lead With Water Reaction.
From www.esseltek.com
Lead's Impact On Water Quality Essel Environmental Lead With Water Reaction Wrappings for food, tobacco, and other products;. Under normal conditions lead does not react with water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Lead reacts with air, in the. Lead With Water Reaction.
From exocoyzqy.blob.core.windows.net
Lead In Water Chemical Equation at Gordon Maxwell blog Lead With Water Reaction All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Wrappings for food, tobacco, and other products;. The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather. Lead With Water Reaction.
From halifaxwater.ca
Lead & Water Quality Halifax Water Lead With Water Reaction Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Wrappings for food, tobacco, and other products;. The layer is most likely lead hydroxy carbonate [8]. Lead reacts with air, in the presence. Lead With Water Reaction.
From waterbadge.com
7 Symptoms of Lead in Water Lead With Water Reaction The layer is most likely lead hydroxy carbonate [8]. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. However, when lead comes in contact with moist air reactivity with water increases. All of group 1. Lead With Water Reaction.
From tataandhoward.com
6 Facts About Lead In Drinking Water Tata & Howard Lead With Water Reaction Type metal and other alloys; The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Throughout history, lead has been used to make water and sewer pipes; Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. The layer is most likely. Lead With Water Reaction.
From www.youtube.com
Double displacement HCl + Pb(NO3)2 Hydrochloric acid + Lead(ii Lead With Water Reaction The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Throughout history, lead has been used to make water and sewer pipes; Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. However, when lead comes in contact with moist air reactivity with water. Lead With Water Reaction.
From zabir.ru
Water reaction Lead With Water Reaction Under normal conditions lead does not react with water. The layer is most likely lead hydroxy carbonate [8]. However, when lead comes in contact with moist air reactivity with water increases. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. Wrappings for food, tobacco, and other products;. Lead(ii) ion reacts with. Lead With Water Reaction.
From isingsculliganwater.com
What to Do About Lead in Your Water? Lead With Water Reaction However, when lead comes in contact with moist air reactivity with water increases. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Throughout history, lead has been used to make water. Lead With Water Reaction.
From etrlabs.com
Is My Water Contaminated With Lead? ETR Laboratories, Inc. Lead With Water Reaction The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Wrappings for food, tobacco, and other products;. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride. Lead With Water Reaction.
From digital.library.unt.edu
Lithiumlead/water reaction experiments and analysis UNT Digital Library Lead With Water Reaction Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. Wrappings for food, tobacco, and other products;. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Type metal and other alloys; However, when lead comes in contact with moist air reactivity with water increases. Under. Lead With Water Reaction.
From www.cleanwater.org
Lead and Drinking Water Clean Water Action Lead With Water Reaction Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. Wrappings for food, tobacco, and other products;. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or. Lead With Water Reaction.
From www.compoundchem.com
Chemical Reactions Lead Iodide & 'Golden Rain' Compound Interest Lead With Water Reaction The layer is most likely lead hydroxy carbonate [8]. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Throughout history, lead has been used to make water and sewer pipes; Under normal conditions lead does not react with water. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic. Lead With Water Reaction.
From www.who.int
Lead in drinkingwater Health risks, monitoring and corrective actions Lead With Water Reaction Under normal conditions lead does not react with water. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Type metal and other alloys; All of group 1 elements —lithium, sodium, potassium,. Lead With Water Reaction.
From www.tapwatercompany.com
Lead in Water What You Need to Know Lead With Water Reaction The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: However, when lead comes in contact with moist air reactivity with water increases. Wrappings for food, tobacco, and other products;. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: All. Lead With Water Reaction.
From www.youtube.com
Equation for PbSO4 + H2O Lead (II) sulfate + Water YouTube Lead With Water Reaction Throughout history, lead has been used to make water and sewer pipes; The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Wrappings for food, tobacco, and other products;. Under normal conditions lead does not react with water. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt,. Lead With Water Reaction.
From www.youtube.com
Lead in drinking water YouTube Lead With Water Reaction However, when lead comes in contact with moist air reactivity with water increases. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Throughout history, lead has been used to make water and sewer pipes;. Lead With Water Reaction.
From www.numerade.com
SOLVED Write the reaction when solid lead (II) cyanide is put into Lead With Water Reaction Type metal and other alloys; The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: However, when lead comes in contact with moist air reactivity with water increases. Lead reacts with. Lead With Water Reaction.
From netsolwater.com
How do you remove lead from Industrial waste water Lead With Water Reaction Wrappings for food, tobacco, and other products;. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Type metal and other alloys; Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just. Lead With Water Reaction.
From angelwater.com
Lead in Water? How to Get It Out Angel Water, Inc. Lead With Water Reaction Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Wrappings for food, tobacco, and other products;. Throughout history, lead has been used to make water and sewer pipes; Type metal and other alloys; The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium,. Lead With Water Reaction.
From byjus.com
What are chemical reactions taking place inside a lead accumulator when Lead With Water Reaction Type metal and other alloys; Wrappings for food, tobacco, and other products;. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Under normal conditions lead does not react with water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. The general reaction of. Lead With Water Reaction.
From www.cleanwateraction.org
Lead and Drinking Water Clean Water Action Lead With Water Reaction However, when lead comes in contact with moist air reactivity with water increases. The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water. Lead With Water Reaction.
From mammothmemory.net
Leads reaction with hydrochloric acid is very slow indeed Lead With Water Reaction The general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: The layer is most likely lead hydroxy carbonate [8]. Lead reacts with air, in the presence of moist and carbon dioxide, forming a passivating layer. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than. Lead With Water Reaction.
From www.nrdc.org
Causes and Effects of Lead in Water Lead With Water Reaction However, when lead comes in contact with moist air reactivity with water increases. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. Throughout history, lead has been used to make water and sewer pipes; Type metal and other alloys; Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt,. Lead With Water Reaction.