Does Graphite Absorb Heat . As with all elements, it increases with temperature, with the following relationship (t in. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. Diamond is better at transferring heat. Graphite has the material property of heat absorption and dispersion and is applied in heat. Graphite conducts electricity(only across a layer) where as diamond does not.
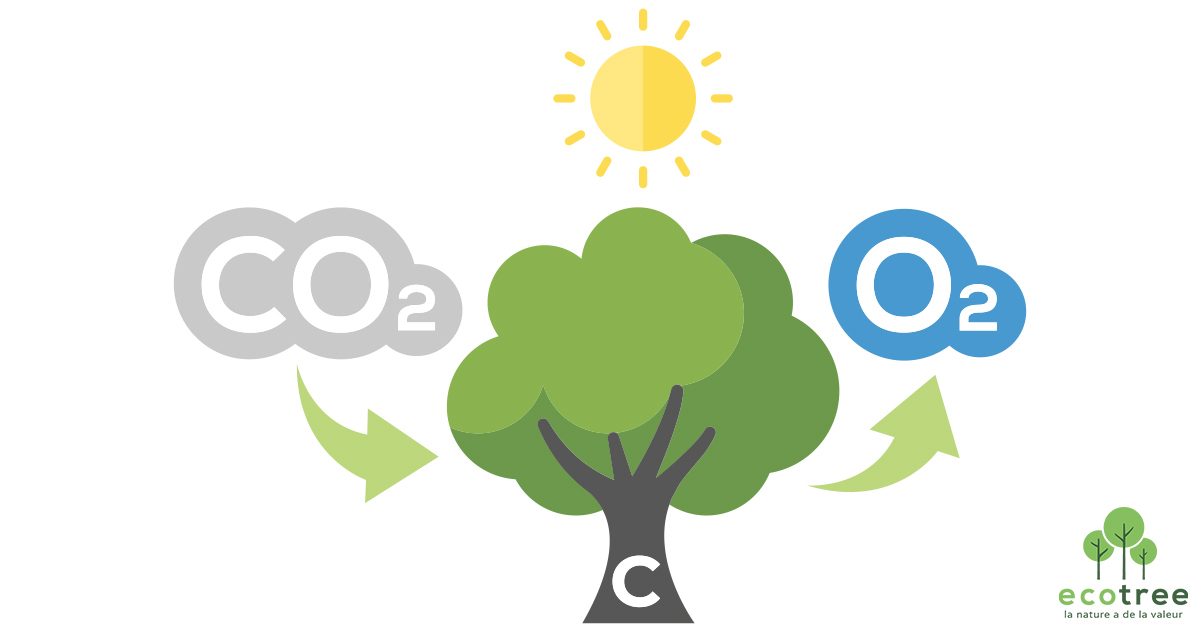
from ecotree.green
Graphite has the material property of heat absorption and dispersion and is applied in heat. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. As with all elements, it increases with temperature, with the following relationship (t in. Diamond is better at transferring heat. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. Graphite conducts electricity(only across a layer) where as diamond does not.
How much CO2 does a tree absorb? Let’s get carbon curious!
Does Graphite Absorb Heat The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. As with all elements, it increases with temperature, with the following relationship (t in. Diamond is better at transferring heat. Graphite has the material property of heat absorption and dispersion and is applied in heat. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. Graphite conducts electricity(only across a layer) where as diamond does not.
From materialcampusgloria.z21.web.core.windows.net
Heat Absorption Color Chart Does Graphite Absorb Heat Diamond is better at transferring heat. Graphite has the material property of heat absorption and dispersion and is applied in heat. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the. Does Graphite Absorb Heat.
From whowhatwhy.org
Climate Questions How Does Carbon Dioxide Trap Heat? WhoWhatWhy Does Graphite Absorb Heat Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. The oxidation characteristics of any graphite at the. Does Graphite Absorb Heat.
From byjus.com
32.Why graphite conduct electricity but diamond doesn't while both are Does Graphite Absorb Heat The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. Graphite has the material property of heat absorption and dispersion and is applied in heat. Diamond is. Does Graphite Absorb Heat.
From mwi-inc.com
What is Graphite? MWI, Inc. Does Graphite Absorb Heat Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Graphite has the material property of heat absorption and dispersion and is applied in heat. Natural graphite. Does Graphite Absorb Heat.
From energytheory.com
How Much Carbon Dioxide Does a Tree Absorb Per Day? Energy Theory Does Graphite Absorb Heat Graphite conducts electricity(only across a layer) where as diamond does not. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. As with all elements, it increases with temperature, with. Does Graphite Absorb Heat.
From itechguidessc.pages.dev
Does Carbon Fiber Conduct Electricity itechguides Does Graphite Absorb Heat Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. As with all elements, it increases with temperature, with the following relationship (t in. Graphite has the material property of. Does Graphite Absorb Heat.
From uk.movies.yahoo.com
Climate Questions How does carbon dioxide trap heat? Does Graphite Absorb Heat Graphite has the material property of heat absorption and dispersion and is applied in heat. Graphite conducts electricity(only across a layer) where as diamond does not. Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. As with all elements, it increases with temperature, with the following relationship (t in. Natural graphite. Does Graphite Absorb Heat.
From www.nuclear-power.com
Carbon Specific Heat, Latent Heat of Fusion, Latent Heat of Does Graphite Absorb Heat Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. As with all elements, it increases with temperature, with the following relationship (t in. Graphite conducts electricity(only across a layer) where as diamond does not. Graphite has the material property. Does Graphite Absorb Heat.
From fyoryrbtc.blob.core.windows.net
Do Older Trees Absorb More Co2 at Beverly Goodman blog Does Graphite Absorb Heat Diamond is better at transferring heat. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. Graphite conducts electricity(only. Does Graphite Absorb Heat.
From za.pinterest.com
Greenhouse gases are gases in the atmosphere that absorb and emit heat Does Graphite Absorb Heat Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. Diamond is better at transferring heat. Graphite is a mineral that forms. Does Graphite Absorb Heat.
From fyoqvcrsh.blob.core.windows.net
How Much Carbon Dioxide Does A Tree Absorb at Nathan Land blog Does Graphite Absorb Heat Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. As with all elements, it increases with temperature, with the following relationship (t in. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a. Does Graphite Absorb Heat.
From byjus.com
43.Why does diamond not have free electrons while graphite has? Does Graphite Absorb Heat Graphite has the material property of heat absorption and dispersion and is applied in heat. Graphite conducts electricity(only across a layer) where as diamond does not. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Natural graphite is an excellent conductor of heat and electricity, stable over a. Does Graphite Absorb Heat.
From sciencenotes.org
Adsorption vs Absorption Differences and Examples Does Graphite Absorb Heat Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. The oxidation characteristics of any graphite at the. Does Graphite Absorb Heat.
From byjus.com
Does graphite conduct electricity? Does Graphite Absorb Heat The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. Graphite conducts electricity(only across a layer) where as diamond does not. Graphite has the material property of. Does Graphite Absorb Heat.
From cleantechnica.com
Forests Absorb Twice As Much Carbon As They Emit Each Year CleanTechnica Does Graphite Absorb Heat Diamond is better at transferring heat. Graphite has the material property of heat absorption and dispersion and is applied in heat. Graphite conducts electricity(only across a layer) where as diamond does not. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Natural graphite is an excellent conductor of. Does Graphite Absorb Heat.
From www.chandlerpond.org
Let’s talk Climate Change Which trees absorb the most carbon dioxide Does Graphite Absorb Heat As with all elements, it increases with temperature, with the following relationship (t in. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. Diamond is better at transferring heat. Graphite conducts electricity(only across a layer) where as diamond does. Does Graphite Absorb Heat.
From fyonukyyh.blob.core.windows.net
Does Plant Absorb Oxygen at Tina King blog Does Graphite Absorb Heat The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. Graphite is a mineral that forms when carbon is. Does Graphite Absorb Heat.
From www.scienceabc.com
How Does Graphite Get Inside A Pencil? » ScienceABC Does Graphite Absorb Heat Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. Graphite has the material property of heat absorption and dispersion and is applied in heat. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point. Does Graphite Absorb Heat.
From www.linkedin.com
How does the graphite sheet help the laptop to dissipate heat? Does Graphite Absorb Heat Graphite conducts electricity(only across a layer) where as diamond does not. Graphite has the material property of heat absorption and dispersion and is applied in heat. Diamond is better at transferring heat. As with all elements, it increases with temperature, with the following relationship (t in. Graphite’s relatively high specific heat capacity means that it can absorb a significant amount. Does Graphite Absorb Heat.
From opengeology.org
11 Metamorphic Equilibria Open Petrology Does Graphite Absorb Heat Diamond is better at transferring heat. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Graphite conducts electricity(only across a layer) where as diamond does not.. Does Graphite Absorb Heat.
From netsolwater.com
How does Activated Carbon work? Function of Activated Carbon Does Graphite Absorb Heat The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Graphite has the material property of heat absorption and dispersion and is applied in heat. Diamond is better at transferring heat. As with all elements, it increases with temperature, with the following relationship (t in. Graphite’s relatively high specific. Does Graphite Absorb Heat.
From www.researchgate.net
4.1 Energetic situation for the transformation of graphite to diamond Does Graphite Absorb Heat Diamond is better at transferring heat. As with all elements, it increases with temperature, with the following relationship (t in. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. Graphite conducts electricity(only across a layer) where as diamond does. Does Graphite Absorb Heat.
From www.youtube.com
How Much CO2 Does a Tree Absorb? YouTube Does Graphite Absorb Heat Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. Graphite’s relatively high specific heat capacity means that. Does Graphite Absorb Heat.
From globalocean.noaa.gov
Global Atmospheric Carbon Dioxide Levels Continue to Rise Global Does Graphite Absorb Heat Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. Graphite conducts electricity(only across a layer) where as diamond does not. Graphite has the material property of heat absorption and dispersion and is applied in heat. As with all elements, it increases with temperature, with the following relationship. Does Graphite Absorb Heat.
From giosvwueu.blob.core.windows.net
Do Plants Absorb Carbon Dioxide From The Soil at Ernesto Brouillard blog Does Graphite Absorb Heat Graphite has the material property of heat absorption and dispersion and is applied in heat. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Graphite’s relatively. Does Graphite Absorb Heat.
From thurne.se
Graphite tube Heat Exchanger THURNE Does Graphite Absorb Heat Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. Graphite conducts electricity(only across a layer) where as diamond does not. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. The. Does Graphite Absorb Heat.
From energytheory.com
How Much Carbon Dioxide Does a Tree Absorb Per Day? Energy Theory Does Graphite Absorb Heat Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. Diamond is better at transferring heat. Graphite has the material property of. Does Graphite Absorb Heat.
From tutormate.in
Allotropes of carbon Tutormate Does Graphite Absorb Heat Diamond is better at transferring heat. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. Graphite is a mineral that forms when carbon is subjected to heat and pressure in. Does Graphite Absorb Heat.
From phytocat.org
How Much Co2 Do Plants Absorb Globally? Does Graphite Absorb Heat Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material with a high melting point of 3650 °c. Diamond is better at transferring heat. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. As with. Does Graphite Absorb Heat.
From ar.inspiredpencil.com
Carbon Dioxide Cycle Ocean Does Graphite Absorb Heat Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. Diamond is better at transferring heat. As with all elements, it increases with temperature, with the following relationship (t in. Natural graphite is an excellent conductor of heat and electricity, stable over a broad range of temperatures, and a highly refractory material. Does Graphite Absorb Heat.
From gioucoqzn.blob.core.windows.net
Do Trees Absorb Carbon Dioxide In Winter at Brian Deutsch blog Does Graphite Absorb Heat As with all elements, it increases with temperature, with the following relationship (t in. Graphite conducts electricity(only across a layer) where as diamond does not. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Graphite has the material property of heat absorption and dispersion and is applied in. Does Graphite Absorb Heat.
From techiescientist.com
Does Graphite Conduct Electricity? Techiescientist Does Graphite Absorb Heat Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. As with all elements, it increases with temperature, with the following relationship (t in. Natural graphite is an excellent conductor. Does Graphite Absorb Heat.
From ecotree.green
How much CO2 does a tree absorb? Let’s get carbon curious! Does Graphite Absorb Heat Graphite conducts electricity(only across a layer) where as diamond does not. Graphite’s relatively high specific heat capacity means that it can absorb a significant amount of heat without experiencing. Diamond is better at transferring heat. As with all elements, it increases with temperature, with the following relationship (t in. The oxidation characteristics of any graphite at the same temperature and. Does Graphite Absorb Heat.
From onetreeplanted.org
How Much CO2 Does A Tree Absorb? One Tree Planted Does Graphite Absorb Heat Graphite has the material property of heat absorption and dispersion and is applied in heat. Graphite conducts electricity(only across a layer) where as diamond does not. Diamond is better at transferring heat. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Natural graphite is an excellent conductor of. Does Graphite Absorb Heat.
From www.numerade.com
Gaseous water and carbon dioxide each absorb infrared radiation. Does Does Graphite Absorb Heat Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper mantle. Diamond is better at transferring heat. The oxidation characteristics of any graphite at the same temperature and atmospheric conditions are dependent on the amount of impurities in. Graphite has the material property of heat absorption and dispersion and. Does Graphite Absorb Heat.