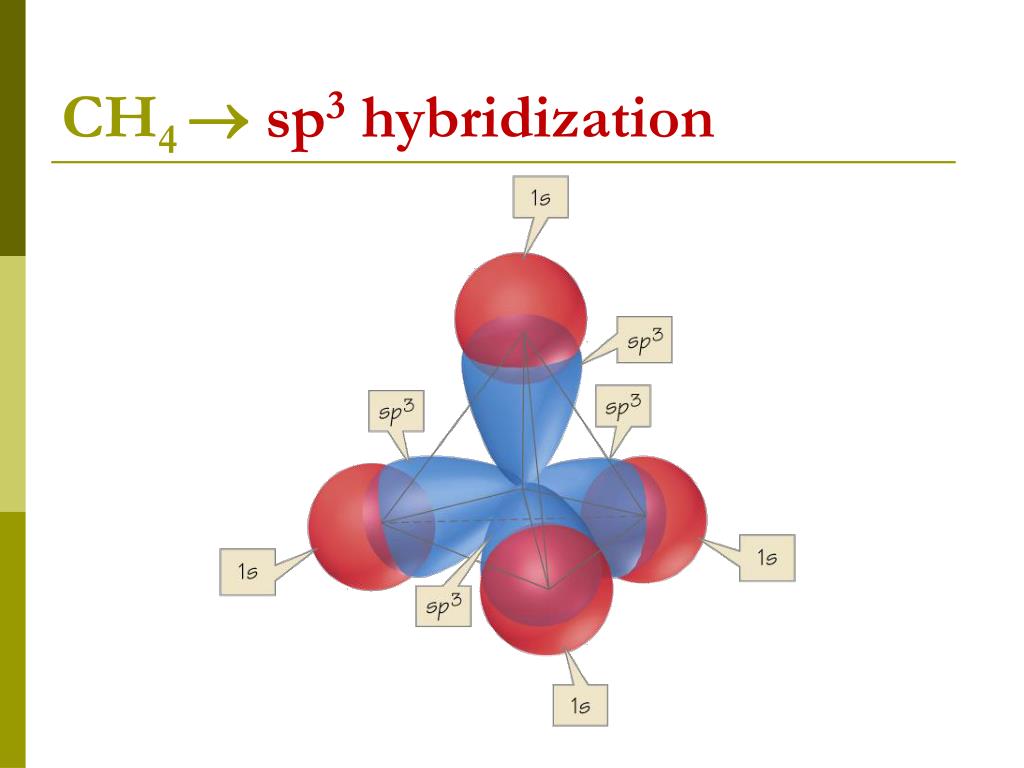
Explain Hybridization In Ch4 . The type of hybridization involved with ch4 is sp 3. — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. We will discuss in detail how this hybridization occurs below. the electrons rearrange themselves again in a process called hybridization. The electrons rearrange themselves again in a process called hybridisation. valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h 2. — to understand the hybridization of methane (ch 4), we have to examine. to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. — to find the hybridization for ch4 we’ll first determine the steric. This reorganizes the electrons into four identical hybrid orbitals called sp 3. — this article discusses the hybridization of ch4 (methane), its molecular geometry, bond angles, and key points to remember.
from www.slideserve.com
The type of hybridization involved with ch4 is sp 3. — to find the hybridization for ch4 we’ll first determine the steric. We will discuss in detail how this hybridization occurs below. to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. — to understand the hybridization of methane (ch 4), we have to examine. valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h 2. This reorganizes the electrons into four identical hybrid orbitals called sp 3. — this article discusses the hybridization of ch4 (methane), its molecular geometry, bond angles, and key points to remember. the electrons rearrange themselves again in a process called hybridization.
PPT Unit 04 BONDING PowerPoint Presentation, free download ID2109992
Explain Hybridization In Ch4 — this article discusses the hybridization of ch4 (methane), its molecular geometry, bond angles, and key points to remember. The type of hybridization involved with ch4 is sp 3. The electrons rearrange themselves again in a process called hybridisation. to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. This reorganizes the electrons into four identical hybrid orbitals called sp 3. the electrons rearrange themselves again in a process called hybridization. — to find the hybridization for ch4 we’ll first determine the steric. We will discuss in detail how this hybridization occurs below. valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h 2. — this article discusses the hybridization of ch4 (methane), its molecular geometry, bond angles, and key points to remember. — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. — to understand the hybridization of methane (ch 4), we have to examine.
From www.youtube.com
Chemistry 11Chapter 4 Chemical Bonding &Molecular Structure Explain Hybridization In Ch4 to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. — to understand the hybridization of methane (ch 4), we have to examine. the electrons rearrange themselves again in a process called. Explain Hybridization In Ch4.
From brainly.in
Write hybridization of CH4, PCl5 and SF4. Brainly.in Explain Hybridization In Ch4 to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. The type of hybridization involved with ch4 is sp 3. The electrons rearrange themselves again in a process called hybridisation. valence bond theory's. Explain Hybridization In Ch4.
From pt.slideshare.net
Tang 08 hybridization Explain Hybridization In Ch4 This reorganizes the electrons into four identical hybrid orbitals called sp 3. valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h 2. We will discuss in detail how this hybridization occurs below. The type of hybridization involved with ch4 is sp 3. the electrons. Explain Hybridization In Ch4.
From chemistryskills.com
Orbital Hybridization Chemistry Skills Explain Hybridization In Ch4 This reorganizes the electrons into four identical hybrid orbitals called sp 3. The electrons rearrange themselves again in a process called hybridisation. — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. We will discuss. Explain Hybridization In Ch4.
From techiescientist.com
CH4 Lewis Structure, Molecular Geometry, and Hybridization Explain Hybridization In Ch4 — to find the hybridization for ch4 we’ll first determine the steric. — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. The type of hybridization involved with ch4 is sp 3. to. Explain Hybridization In Ch4.
From www.chemistrylibrary.org
Types of Hybridization Explain Hybridization In Ch4 — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. — to understand the hybridization of methane (ch 4), we have to examine. valence bond theory's use of overlapping atomic orbitals to explain. Explain Hybridization In Ch4.
From chemwiki.ucdavis.edu
10.3 Hybridization of Atomic Orbitals Chemwiki Explain Hybridization In Ch4 The electrons rearrange themselves again in a process called hybridisation. to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. the electrons rearrange themselves again in a process called hybridization. — in. Explain Hybridization In Ch4.
From chem.libretexts.org
Hybridization Chemistry LibreTexts Explain Hybridization In Ch4 — this article discusses the hybridization of ch4 (methane), its molecular geometry, bond angles, and key points to remember. — to find the hybridization for ch4 we’ll first determine the steric. the electrons rearrange themselves again in a process called hybridization. to explain the bonding of carbon and other atoms that cannot fit into the simple. Explain Hybridization In Ch4.
From classnotes.org.in
Hybridisation Chemical Bonding and Molecular Structure, Chemistry Explain Hybridization In Ch4 — to find the hybridization for ch4 we’ll first determine the steric. to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. — this article discusses the hybridization of ch4 (methane), its. Explain Hybridization In Ch4.
From www.pinterest.com.mx
Orbital Hybridization "Cheat Sheet"? + Example Chemistry lessons Explain Hybridization In Ch4 — this article discusses the hybridization of ch4 (methane), its molecular geometry, bond angles, and key points to remember. the electrons rearrange themselves again in a process called hybridization. — to find the hybridization for ch4 we’ll first determine the steric. to explain the bonding of carbon and other atoms that cannot fit into the simple. Explain Hybridization In Ch4.
From www.numerade.com
SOLVED What is the hybridization of carbon in the CH4 molecule? 3 sp Explain Hybridization In Ch4 We will discuss in detail how this hybridization occurs below. the electrons rearrange themselves again in a process called hybridization. — to find the hybridization for ch4 we’ll first determine the steric. The type of hybridization involved with ch4 is sp 3. The electrons rearrange themselves again in a process called hybridisation. — in order to understand. Explain Hybridization In Ch4.
From techiescientist.com
CH4 Lewis Structure, Molecular Geometry, and Hybridization Explain Hybridization In Ch4 valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h 2. to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond. Explain Hybridization In Ch4.
From www.studypool.com
SOLUTION Hybridization in ch4 using group theory Studypool Explain Hybridization In Ch4 — to find the hybridization for ch4 we’ll first determine the steric. We will discuss in detail how this hybridization occurs below. This reorganizes the electrons into four identical hybrid orbitals called sp 3. the electrons rearrange themselves again in a process called hybridization. — to understand the hybridization of methane (ch 4), we have to examine.. Explain Hybridization In Ch4.
From brainly.in
what is hybridisation? explain hybridization in methane (CH4) Brainly.in Explain Hybridization In Ch4 — this article discusses the hybridization of ch4 (methane), its molecular geometry, bond angles, and key points to remember. — to find the hybridization for ch4 we’ll first determine the steric. This reorganizes the electrons into four identical hybrid orbitals called sp 3. — to understand the hybridization of methane (ch 4), we have to examine. . Explain Hybridization In Ch4.
From ar.inspiredpencil.com
Ch4 Hybridization Explain Hybridization In Ch4 — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. This reorganizes the electrons into four identical hybrid orbitals called sp 3. — to find the hybridization for ch4 we’ll first determine the steric.. Explain Hybridization In Ch4.
From brainly.in
what is hybridisation? explain hybridization in methane (CH4) Brainly.in Explain Hybridization In Ch4 — to find the hybridization for ch4 we’ll first determine the steric. — this article discusses the hybridization of ch4 (methane), its molecular geometry, bond angles, and key points to remember. The electrons rearrange themselves again in a process called hybridisation. the electrons rearrange themselves again in a process called hybridization. This reorganizes the electrons into four. Explain Hybridization In Ch4.
From www.geeksforgeeks.org
Hybridization Definition, Types, Rules, Examples Explain Hybridization In Ch4 valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h 2. This reorganizes the electrons into four identical hybrid orbitals called sp 3. The electrons rearrange themselves again in a process called hybridisation. — in order to understand the hybridization of ch 4 (methane), we. Explain Hybridization In Ch4.
From ar.inspiredpencil.com
Ch4 Hybridization Explain Hybridization In Ch4 — to understand the hybridization of methane (ch 4), we have to examine. — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. We will discuss in detail how this hybridization occurs below. . Explain Hybridization In Ch4.
From gamesmartz.com
Hybridization Definition & Image GameSmartz Explain Hybridization In Ch4 the electrons rearrange themselves again in a process called hybridization. We will discuss in detail how this hybridization occurs below. — to understand the hybridization of methane (ch 4), we have to examine. This reorganizes the electrons into four identical hybrid orbitals called sp 3. valence bond theory's use of overlapping atomic orbitals to explain how chemical. Explain Hybridization In Ch4.
From learningschoolarditata5.z21.web.core.windows.net
Hybrid Orbitals Of Ch4 Explain Hybridization In Ch4 The electrons rearrange themselves again in a process called hybridisation. This reorganizes the electrons into four identical hybrid orbitals called sp 3. the electrons rearrange themselves again in a process called hybridization. — this article discusses the hybridization of ch4 (methane), its molecular geometry, bond angles, and key points to remember. The type of hybridization involved with ch4. Explain Hybridization In Ch4.
From www.studypool.com
SOLUTION Hybridization in ch4 using group theory Studypool Explain Hybridization In Ch4 valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h 2. to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond. Explain Hybridization In Ch4.
From byjus.com
Hybridization of Carbon Molecular Geometry and Bond Angles Explain Hybridization In Ch4 — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. — to find the hybridization for ch4 we’ll first determine the steric. — this article discusses the hybridization of ch4 (methane), its molecular. Explain Hybridization In Ch4.
From www.slideserve.com
PPT Valence Bond Theory PowerPoint Presentation, free download ID Explain Hybridization In Ch4 valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h 2. — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process.. Explain Hybridization In Ch4.
From ar.inspiredpencil.com
Ch4 Hybridization Explain Hybridization In Ch4 We will discuss in detail how this hybridization occurs below. — this article discusses the hybridization of ch4 (methane), its molecular geometry, bond angles, and key points to remember. The electrons rearrange themselves again in a process called hybridisation. the electrons rearrange themselves again in a process called hybridization. to explain the bonding of carbon and other. Explain Hybridization In Ch4.
From byjus.com
Hybridization of CH4 (Methane) Hybridization of Carbon in CH4 Explain Hybridization In Ch4 This reorganizes the electrons into four identical hybrid orbitals called sp 3. We will discuss in detail how this hybridization occurs below. valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h 2. — to understand the hybridization of methane (ch 4), we have to. Explain Hybridization In Ch4.
From www.slideserve.com
PPT Unit 04 BONDING PowerPoint Presentation, free download ID2109992 Explain Hybridization In Ch4 — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. the electrons rearrange themselves again in a process called hybridization. — this article discusses the hybridization of ch4 (methane), its molecular geometry, bond. Explain Hybridization In Ch4.
From chemistryteory.blogspot.com
chemistry Hybridization sp3 hybridization in Methane Explain Hybridization In Ch4 valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h 2. the electrons rearrange themselves again in a process called hybridization. — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are. Explain Hybridization In Ch4.
From mavink.com
Ch4 Orbital Diagram Explain Hybridization In Ch4 The electrons rearrange themselves again in a process called hybridisation. the electrons rearrange themselves again in a process called hybridization. The type of hybridization involved with ch4 is sp 3. — to find the hybridization for ch4 we’ll first determine the steric. This reorganizes the electrons into four identical hybrid orbitals called sp 3. valence bond theory's. Explain Hybridization In Ch4.
From www.studypool.com
SOLUTION Hybridization in ch4 using group theory Studypool Explain Hybridization In Ch4 valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h 2. to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond. Explain Hybridization In Ch4.
From www.slideshare.net
Tang 08 hybridization Explain Hybridization In Ch4 to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are. Explain Hybridization In Ch4.
From www.youtube.com
Hybridization of CH4 (Methane) YouTube Explain Hybridization In Ch4 — to understand the hybridization of methane (ch 4), we have to examine. The type of hybridization involved with ch4 is sp 3. to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory.. Explain Hybridization In Ch4.
From ar.inspiredpencil.com
Ch4 Hybridization Explain Hybridization In Ch4 to explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h. Explain Hybridization In Ch4.
From schematicmemberfdiche.z22.web.core.windows.net
Mo Diagram For Ch4 Explain Hybridization In Ch4 — this article discusses the hybridization of ch4 (methane), its molecular geometry, bond angles, and key points to remember. — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. We will discuss in detail. Explain Hybridization In Ch4.
From ar.inspiredpencil.com
Ch4 Hybridization Explain Hybridization In Ch4 The electrons rearrange themselves again in a process called hybridisation. — in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. — to understand the hybridization of methane (ch 4), we have to examine. . Explain Hybridization In Ch4.
From www.studypool.com
SOLUTION Hybridization in ch4 using group theory Studypool Explain Hybridization In Ch4 valence bond theory's use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as h 2. The type of hybridization involved with ch4 is sp 3. the electrons rearrange themselves again in a process called hybridization. — to find the hybridization for ch4 we’ll first determine the steric. . Explain Hybridization In Ch4.