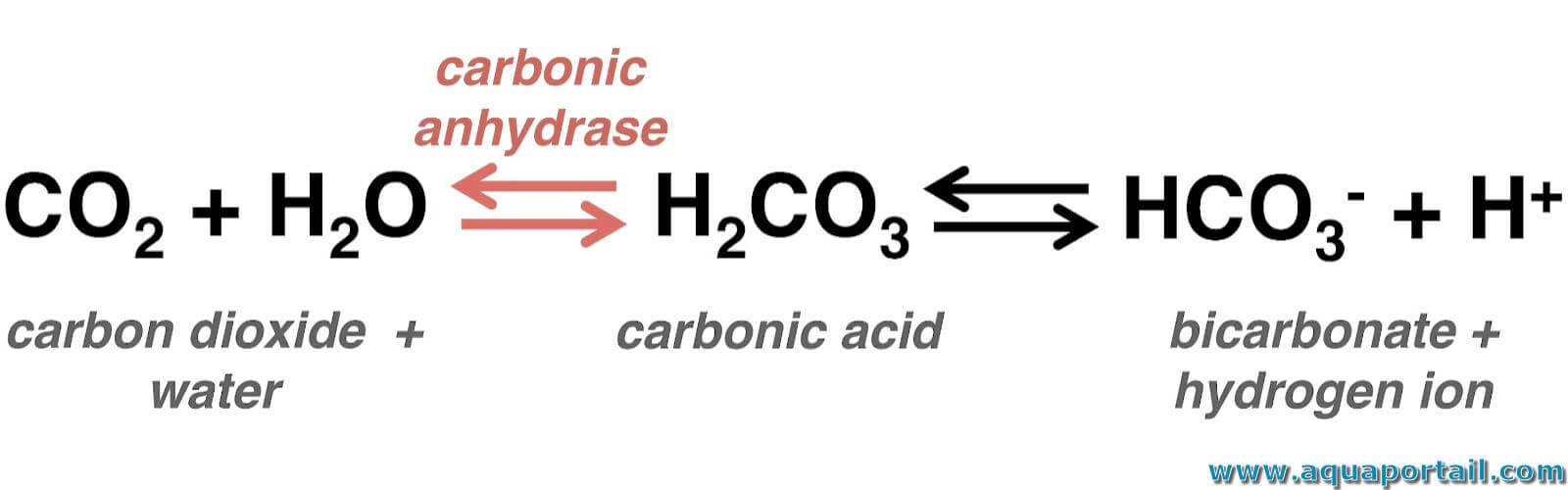
Carbonic Acid Body . These reversible reactions function as a buffer system. The buffer that maintains the ph of human blood involves carbonic acid (h 2. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. The loss of co 2 from the body reduces blood levels of carbonic acid and thereby adjusts the ph upward, toward normal levels. Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16).
from www.aquaportail.com
The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). The loss of co 2 from the body reduces blood levels of carbonic acid and thereby adjusts the ph upward, toward normal levels. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. These reversible reactions function as a buffer system. Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The buffer that maintains the ph of human blood involves carbonic acid (h 2.
Anhydride carbonique définition et explications
Carbonic Acid Body These reversible reactions function as a buffer system. The buffer that maintains the ph of human blood involves carbonic acid (h 2. The loss of co 2 from the body reduces blood levels of carbonic acid and thereby adjusts the ph upward, toward normal levels. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. These reversible reactions function as a buffer system. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16).
From www.visionlearning.com
Acids and Bases II Biology Visionlearning Carbonic Acid Body Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. The respiratory system contributes to the balance of acids and bases in the body. Carbonic Acid Body.
From alchetron.com
Carbonic acid Alchetron, The Free Social Encyclopedia Carbonic Acid Body The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). The buffer that maintains the ph of human blood involves carbonic acid (h 2. The loss of co 2 from the body reduces blood levels of carbonic acid and thereby adjusts the ph upward, toward normal. Carbonic Acid Body.
From www.shutterstock.com
Carbonic Acid H2co3 Molecule Chemical Structure Stock Illustration Carbonic Acid Body The buffer that maintains the ph of human blood involves carbonic acid (h 2. These reversible reactions function as a buffer system. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive. Carbonic Acid Body.
From www.dreamstime.com
Infographic of the Molecule of Carbonic Acid Stock Vector Carbonic Acid Body Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The buffer that maintains the ph of human blood involves carbonic acid (h 2. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. Carbonic Acid Body.
From www.mdpi.com
IJMS Free FullText Role of Carbonic Anhydrases and Inhibitors in Carbonic Acid Body These reversible reactions function as a buffer system. Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The buffer that maintains the ph of human blood involves carbonic acid (h 2. The respiratory system contributes to the balance of acids and bases in the body. Carbonic Acid Body.
From www.pinterest.com
Image result for bicarbonate buffer system Medical student motivation Carbonic Acid Body These reversible reactions function as a buffer system. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The respiratory system contributes. Carbonic Acid Body.
From straighthealthcare.com
Normal acidbase physiology illustration Carbonic Acid Body The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). These reversible reactions function as a buffer system. Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The loss of co. Carbonic Acid Body.
From www.alamy.com
Carbonic acid molecule. Formed when carbon dioxide is dissolved in Carbonic Acid Body The buffer that maintains the ph of human blood involves carbonic acid (h 2. These reversible reactions function as a buffer system. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive. Carbonic Acid Body.
From www.dreamstime.com
Carbonic Acid, Molecular Structures, Hydroxyformic Acid, 3d Model Carbonic Acid Body The buffer that maintains the ph of human blood involves carbonic acid (h 2. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively. Carbonic Acid Body.
From www.thoughtco.com
10 Common Acids and Chemical Structures Carbonic Acid Body The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. The loss of co 2 from the body reduces blood levels of carbonic acid. Carbonic Acid Body.
From journals.physiology.org
In the pink and why so blue? A metabolic acidosis “shockandawe Carbonic Acid Body Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The loss of co 2 from the body reduces blood levels of carbonic acid and thereby adjusts the ph upward, toward normal levels. The respiratory system contributes to the balance of acids and bases in the. Carbonic Acid Body.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory Carbonic Acid Body These reversible reactions function as a buffer system. Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The buffer that maintains the ph of human blood involves carbonic acid (h 2. The respiratory system contributes to the balance of acids and bases in the body. Carbonic Acid Body.
From collegedunia.com
Carbonic Acid H2CO3, Structure, Properties & Uses Carbonic Acid Body These reversible reactions function as a buffer system. The loss of co 2 from the body reduces blood levels of carbonic acid and thereby adjusts the ph upward, toward normal levels. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). The buffer that maintains the. Carbonic Acid Body.
From www.slideshare.net
Acid base balance Carbonic Acid Body The buffer that maintains the ph of human blood involves carbonic acid (h 2. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid.. Carbonic Acid Body.
From pressbooks.bccampus.ca
26.4 AcidBase Balance Douglas College Human Anatomy and Physiology Carbonic Acid Body These reversible reactions function as a buffer system. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The buffer that maintains. Carbonic Acid Body.
From www.researchgate.net
Effects of the taste of carbonation and CO2 signaling in gustatory Carbonic Acid Body The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. The loss of co 2 from the body reduces blood levels of carbonic acid and thereby adjusts the ph upward, toward normal levels. The buffer that maintains the ph of human blood involves carbonic acid (h 2. These. Carbonic Acid Body.
From www.dreamstime.com
Carbonic Acid Molecule Icon Stock Illustration Illustration of symbol Carbonic Acid Body Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The buffer that maintains the ph of human blood involves carbonic acid (h 2. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. Carbonic Acid Body.
From www.slideserve.com
PPT Chapter 27, part 2 PowerPoint Presentation, free download ID Carbonic Acid Body The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. These reversible reactions function as a buffer system. The buffer that maintains the ph. Carbonic Acid Body.
From www.numerade.com
Drag the labels onto the diagram to identify the components of the Carbonic Acid Body The buffer that maintains the ph of human blood involves carbonic acid (h 2. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively. Carbonic Acid Body.
From www.slideserve.com
PPT Carbonic AcidBicarbonate Buffering System PowerPoint Carbonic Acid Body The buffer that maintains the ph of human blood involves carbonic acid (h 2. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively. Carbonic Acid Body.
From www.chegg.com
Solved The response to alkalosis caused by the removal of H+ Carbonic Acid Body The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive. Carbonic Acid Body.
From www.vectorstock.com
H2CO3 Carbonic acid molecule Royalty Free Vector Image Carbonic Acid Body These reversible reactions function as a buffer system. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. The buffer that maintains the ph of human blood involves carbonic acid (h 2. Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated. Carbonic Acid Body.
From www.alamy.com
3D image of Carbonic acid skeletal formula molecular chemical Carbonic Acid Body The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). These reversible reactions function as a buffer system. The loss of co 2 from the body reduces blood levels of carbonic acid and thereby adjusts the ph upward, toward normal levels. The buffer that maintains the. Carbonic Acid Body.
From www.thoughtco.com
10 Common Acids and Chemical Structures Carbonic Acid Body The loss of co 2 from the body reduces blood levels of carbonic acid and thereby adjusts the ph upward, toward normal levels. Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The respiratory system contributes to the balance of acids and bases in the. Carbonic Acid Body.
From www.dreamstime.com
Carbonic Acid Molecule Icon Stock Illustration Illustration of Carbonic Acid Body The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). The loss of co 2 from the body reduces blood levels of carbonic acid and thereby adjusts the ph upward, toward normal levels. Bicarbonate ions bind with the h + from introduced acids, while introduced bases. Carbonic Acid Body.
From www.aquaportail.com
Anhydride carbonique définition et explications Carbonic Acid Body The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). These reversible reactions function as a buffer system. The loss of co 2 from. Carbonic Acid Body.
From www.sciencephoto.com
Carbonic acid molecule, illustration Stock Image F027/8280 Carbonic Acid Body Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. The respiratory system contributes to the balance of acids and bases in the body. Carbonic Acid Body.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint Carbonic Acid Body The buffer that maintains the ph of human blood involves carbonic acid (h 2. The loss of co 2 from the body reduces blood levels of carbonic acid and thereby adjusts the ph upward, toward normal levels. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. These. Carbonic Acid Body.
From www.dreamstime.com
Carbonic Acid H2CO3 Molecule Structural Chemical Formula and Stock Carbonic Acid Body Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. These reversible reactions function as a buffer system. The buffer that maintains the ph of human blood involves carbonic acid (h 2. The respiratory system contributes to the balance of acids and bases in the body. Carbonic Acid Body.
From www.shutterstock.com
Carbonic Acid Structural Chemical Formula Molecule Stock Vector Carbonic Acid Body The loss of co 2 from the body reduces blood levels of carbonic acid and thereby adjusts the ph upward, toward normal levels. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). These reversible reactions function as a buffer system. The respiratory system contributes to. Carbonic Acid Body.
From www.dreamstime.com
Carbonic acid stock illustration. Illustration of symbol 212807993 Carbonic Acid Body These reversible reactions function as a buffer system. The buffer that maintains the ph of human blood involves carbonic acid (h 2. Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The respiratory system contributes to the balance of acids and bases in the body. Carbonic Acid Body.
From www.reagent.co.uk
How Do Biological Buffers Work? The Science Blog Carbonic Acid Body These reversible reactions function as a buffer system. Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. The respiratory system contributes to the. Carbonic Acid Body.
From quizlet.com
Bicarbonate Buffer system Diagram Quizlet Carbonic Acid Body Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). The loss of co 2 from the body reduces blood levels of. Carbonic Acid Body.
From www.alamy.com
Carbonic acid (H2CO3) molecule, chemical structure. Found in Stock Carbonic Acid Body These reversible reactions function as a buffer system. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. The buffer that maintains the ph of human blood involves carbonic acid (h 2. The loss of co 2 from the body reduces blood levels of carbonic acid and thereby. Carbonic Acid Body.
From www.dreamstime.com
Carbonic Acid H2CO3 Molecule . it is Also Solution of Carbon Stock Carbonic Acid Body The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (figure 26.16). Bicarbonate ions bind with the h + from introduced acids, while introduced bases receive a donated h + from carbonic acid, effectively neutralising them. These reversible reactions function as a buffer system. The respiratory system contributes. Carbonic Acid Body.