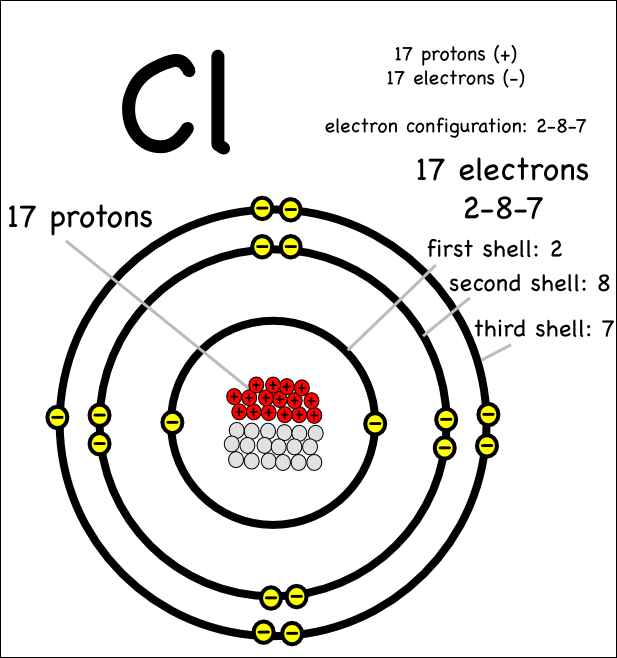
Chlorine Protons Electrons And Valence Electrons . Chlorine can also react with alkenes via the electrophilic addition mechanism. It has an atomic weight of 35.450 and a mass. Therefore, the valence electrons of chlorine are seven. The electron configuration shows that the last shell of chlorine has seven electrons. Group 14 elements, of which carbon is the most important to living systems, have four electrons in their outer shell allowing them. This time two chlorine atoms add to a molecule across the. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. It is an extremely reactive element and a strong oxidising agent: Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. Chlorine is the 17th element of the periodic table so its atomic number is 17. The last shell of a chlorine atom has 7. Valence electrons are the number of electrons present in the outermost shell of an atom. A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. 119 rows valence electrons: The total number of electrons in a valence shell is called valence electrons.
from www.askiitians.com
The last shell of a chlorine atom has 7. Chlorine is the 17th element of the periodic table so its atomic number is 17. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Chlorine can also react with alkenes via the electrophilic addition mechanism. Group 14 elements, of which carbon is the most important to living systems, have four electrons in their outer shell allowing them. A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. The total number of electrons in a valence shell is called valence electrons. Therefore, the valence electrons of chlorine are seven. It is an extremely reactive element and a strong oxidising agent: Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions.
Chlorine Study Material for IIT JEE askIITians
Chlorine Protons Electrons And Valence Electrons It has an atomic weight of 35.450 and a mass. Group 14 elements, of which carbon is the most important to living systems, have four electrons in their outer shell allowing them. Chlorine can also react with alkenes via the electrophilic addition mechanism. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. This time two chlorine atoms add to a molecule across the. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. It is an extremely reactive element and a strong oxidising agent: Valence electrons are the number of electrons present in the outermost shell of an atom. A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. The last shell of a chlorine atom has 7. 119 rows valence electrons: Therefore, the valence electrons of chlorine are seven. It has an atomic weight of 35.450 and a mass. Chlorine is the 17th element of the periodic table so its atomic number is 17. The total number of electrons in a valence shell is called valence electrons. The electron configuration shows that the last shell of chlorine has seven electrons.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Chlorine Protons Electrons And Valence Electrons The last shell of a chlorine atom has 7. Group 14 elements, of which carbon is the most important to living systems, have four electrons in their outer shell allowing them. Chlorine is the 17th element of the periodic table so its atomic number is 17. Chlorine can also react with alkenes via the electrophilic addition mechanism. The total number. Chlorine Protons Electrons And Valence Electrons.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chlorine Protons Electrons And Valence Electrons The electron configuration shows that the last shell of chlorine has seven electrons. It has an atomic weight of 35.450 and a mass. Therefore, the valence electrons of chlorine are seven. It is an extremely reactive element and a strong oxidising agent: A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. Chlorine is the 17th element in the. Chlorine Protons Electrons And Valence Electrons.
From valenceelectrons.com
How to find the Protons, Neutrons and Electrons for Chlorine? Chlorine Protons Electrons And Valence Electrons The electron configuration shows that the last shell of chlorine has seven electrons. A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. Chlorine is the 17th element of the periodic table so its atomic number is 17. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell. Chlorine Protons Electrons And Valence Electrons.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Protons Electrons And Valence Electrons Chlorine is the 17th element of the periodic table so its atomic number is 17. It has an atomic weight of 35.450 and a mass. 119 rows valence electrons: Therefore, the valence electrons of chlorine are seven. Chlorine can also react with alkenes via the electrophilic addition mechanism. It is an extremely reactive element and a strong oxidising agent: A. Chlorine Protons Electrons And Valence Electrons.
From saylordotorg.github.io
Ions Chlorine Protons Electrons And Valence Electrons The electron configuration shows that the last shell of chlorine has seven electrons. Group 14 elements, of which carbon is the most important to living systems, have four electrons in their outer shell allowing them. It is an extremely reactive element and a strong oxidising agent: It has an atomic weight of 35.450 and a mass. Group 17 elements, including. Chlorine Protons Electrons And Valence Electrons.
From elchoroukhost.net
Chlorine Periodic Table Electrons Elcho Table Chlorine Protons Electrons And Valence Electrons 119 rows valence electrons: Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. The electron configuration shows that the last shell of chlorine has. Chlorine Protons Electrons And Valence Electrons.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Protons Electrons And Valence Electrons The total number of electrons in a valence shell is called valence electrons. It has an atomic weight of 35.450 and a mass. Chlorine can also react with alkenes via the electrophilic addition mechanism. Valence electrons are the number of electrons present in the outermost shell of an atom. Chlorine is the 17th element in the periodic table and has. Chlorine Protons Electrons And Valence Electrons.
From www.nuclear-power.com
Chlorine Atomic Number Atomic Mass Density of Chlorine nuclear Chlorine Protons Electrons And Valence Electrons Group 14 elements, of which carbon is the most important to living systems, have four electrons in their outer shell allowing them. Therefore, the valence electrons of chlorine are seven. The last shell of a chlorine atom has 7. A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. It is an extremely reactive element and a strong oxidising. Chlorine Protons Electrons And Valence Electrons.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Protons Electrons And Valence Electrons The total number of electrons in a valence shell is called valence electrons. This time two chlorine atoms add to a molecule across the. The last shell of a chlorine atom has 7. It has an atomic weight of 35.450 and a mass. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. Chlorine Protons Electrons And Valence Electrons.
From pixels.com
Chlorine Electron Configuration Photograph by Chlorine Protons Electrons And Valence Electrons Chlorine can also react with alkenes via the electrophilic addition mechanism. It has an atomic weight of 35.450 and a mass. The last shell of a chlorine atom has 7. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Valence electrons are the number of electrons present in the. Chlorine Protons Electrons And Valence Electrons.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Protons Electrons And Valence Electrons The last shell of a chlorine atom has 7. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. Group 14 elements, of which carbon is the most important to living systems, have four electrons in. Chlorine Protons Electrons And Valence Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Chlorine (Cl Chlorine Protons Electrons And Valence Electrons It has an atomic weight of 35.450 and a mass. A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. Group 14 elements, of which carbon is the most important to living systems, have four electrons in their outer shell allowing them. Valence electrons are the number of electrons present in the outermost shell of an atom. This time. Chlorine Protons Electrons And Valence Electrons.
From www.expii.com
Ions — Definition & Overview Expii Chlorine Protons Electrons And Valence Electrons Chlorine can also react with alkenes via the electrophilic addition mechanism. 119 rows valence electrons: Valence electrons are the number of electrons present in the outermost shell of an atom. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. The electron configuration shows that the last shell of chlorine. Chlorine Protons Electrons And Valence Electrons.
From www.alamy.com
Chlorine (Cl). Diagram of the electron configuration of an atom of Chlorine Protons Electrons And Valence Electrons The electron configuration shows that the last shell of chlorine has seven electrons. Group 14 elements, of which carbon is the most important to living systems, have four electrons in their outer shell allowing them. The total number of electrons in a valence shell is called valence electrons. Chlorine can also react with alkenes via the electrophilic addition mechanism. Chlorine. Chlorine Protons Electrons And Valence Electrons.
From www.numerade.com
SOLVED 'The diagram shows the electron configuration around the Chlorine Protons Electrons And Valence Electrons A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. The total number of electrons in a valence shell is called valence electrons. Valence electrons are the number of electrons present in the outermost shell of an atom. The last shell of a chlorine atom has 7. Therefore, the valence electrons of chlorine are seven. 119 rows valence electrons:. Chlorine Protons Electrons And Valence Electrons.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chlorine Protons Electrons And Valence Electrons A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. The last shell of a chlorine atom has 7. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this. Chlorine Protons Electrons And Valence Electrons.
From www.numerade.com
SOLVED Model 4 Period 3 Elements Sodium Aluminum Chlorine Locate these Chlorine Protons Electrons And Valence Electrons Chlorine can also react with alkenes via the electrophilic addition mechanism. The electron configuration shows that the last shell of chlorine has seven electrons. A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from. Chlorine Protons Electrons And Valence Electrons.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Protons Electrons And Valence Electrons Therefore, the valence electrons of chlorine are seven. It has an atomic weight of 35.450 and a mass. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. A chlorine atom has seventeen protons, eighteen neutrons. Chlorine Protons Electrons And Valence Electrons.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Protons Electrons And Valence Electrons Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. Chlorine is the 17th element of the periodic table so its atomic number is 17. Group 14 elements, of which carbon is the most important to. Chlorine Protons Electrons And Valence Electrons.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Protons Electrons And Valence Electrons Chlorine can also react with alkenes via the electrophilic addition mechanism. Valence electrons are the number of electrons present in the outermost shell of an atom. 119 rows valence electrons: The last shell of a chlorine atom has 7. The total number of electrons in a valence shell is called valence electrons. It has an atomic weight of 35.450 and. Chlorine Protons Electrons And Valence Electrons.
From www.dreamstime.com
Chlorine stock illustration. Illustration of isolated 83614864 Chlorine Protons Electrons And Valence Electrons Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. It has an atomic weight of 35.450 and a mass. This time two chlorine atoms add to a molecule across the. Chlorine is the 17th element. Chlorine Protons Electrons And Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Protons Electrons And Valence Electrons A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. Chlorine is the 17th element of the periodic table so its atomic number is 17. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Group 14 elements, of which carbon is the most important to living systems, have. Chlorine Protons Electrons And Valence Electrons.
From telgurus.co.uk
How to calculate number of neutrons, protons and electrons in Chlorine Chlorine Protons Electrons And Valence Electrons Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. This time two chlorine atoms add to a molecule across the. A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. It has an atomic weight of 35.450 and a mass. 119 rows valence electrons: Valence electrons are the. Chlorine Protons Electrons And Valence Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Chlorine Protons Electrons And Valence Electrons The electron configuration shows that the last shell of chlorine has seven electrons. The last shell of a chlorine atom has 7. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. Valence electrons are the. Chlorine Protons Electrons And Valence Electrons.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Protons Electrons And Valence Electrons Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. The last shell of a chlorine atom has 7. Therefore, the valence electrons of chlorine are seven. This time two chlorine atoms add to a molecule. Chlorine Protons Electrons And Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does ClO2 and ClO2 Have? Chlorine Protons Electrons And Valence Electrons Therefore, the valence electrons of chlorine are seven. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. Chlorine can also react with alkenes via the electrophilic addition mechanism. The last shell of a chlorine atom. Chlorine Protons Electrons And Valence Electrons.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chlorine Protons Electrons And Valence Electrons Chlorine can also react with alkenes via the electrophilic addition mechanism. This time two chlorine atoms add to a molecule across the. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. A chlorine atom has. Chlorine Protons Electrons And Valence Electrons.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Protons Electrons And Valence Electrons This time two chlorine atoms add to a molecule across the. Chlorine can also react with alkenes via the electrophilic addition mechanism. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. 119 rows valence electrons:. Chlorine Protons Electrons And Valence Electrons.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Protons Electrons And Valence Electrons The total number of electrons in a valence shell is called valence electrons. A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. It is an extremely reactive element and a strong oxidising agent: Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. This time two chlorine atoms. Chlorine Protons Electrons And Valence Electrons.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Protons Electrons And Valence Electrons The total number of electrons in a valence shell is called valence electrons. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Chlorine can also react with alkenes via the electrophilic addition mechanism. 119 rows valence electrons: Group 14 elements, of which carbon is the most important to living. Chlorine Protons Electrons And Valence Electrons.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Protons Electrons And Valence Electrons It is an extremely reactive element and a strong oxidising agent: Chlorine is the 17th element of the periodic table so its atomic number is 17. It has an atomic weight of 35.450 and a mass. Group 14 elements, of which carbon is the most important to living systems, have four electrons in their outer shell allowing them. Chlorine is. Chlorine Protons Electrons And Valence Electrons.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons Chlorine Protons Electrons And Valence Electrons Therefore, the valence electrons of chlorine are seven. It is an extremely reactive element and a strong oxidising agent: Chlorine is the 17th element of the periodic table so its atomic number is 17. A chlorine atom has seventeen protons, eighteen neutrons and seventeen electrons. Valence electrons are the number of electrons present in the outermost shell of an atom.. Chlorine Protons Electrons And Valence Electrons.
From favpng.com
Atom Bohr Model Electron Configuration Chlorine, PNG, 1000x1000px, Atom Chlorine Protons Electrons And Valence Electrons The last shell of a chlorine atom has 7. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. 119 rows valence electrons: Chlorine is the 17th element of the periodic table so its atomic number. Chlorine Protons Electrons And Valence Electrons.
From www.expii.com
Valence Electrons — Definition & Importance Expii Chlorine Protons Electrons And Valence Electrons Chlorine can also react with alkenes via the electrophilic addition mechanism. It has an atomic weight of 35.450 and a mass. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. 119 rows valence electrons: The. Chlorine Protons Electrons And Valence Electrons.
From www.askiitians.com
Chlorine Study Material for IIT JEE askIITians Chlorine Protons Electrons And Valence Electrons The electron configuration shows that the last shell of chlorine has seven electrons. This time two chlorine atoms add to a molecule across the. Valence electrons are the number of electrons present in the outermost shell of an atom. It has an atomic weight of 35.450 and a mass. 119 rows valence electrons: Group 17 elements, including fluorine and chlorine,. Chlorine Protons Electrons And Valence Electrons.