Fda Frame Advanced Manufacturing . Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Seek and analyze input to ensure. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. Cohesive regulatory framework for drugs. For example, it approved 2. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology.
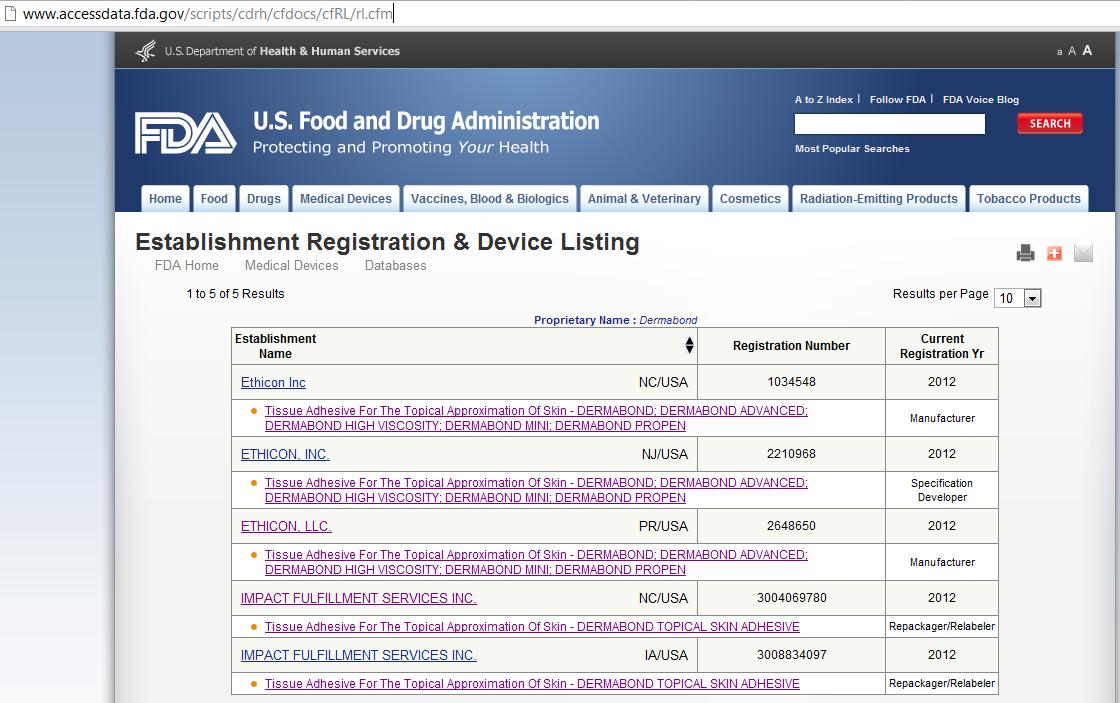
from medicaldeviceacademy.com
For example, it approved 2. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Seek and analyze input to ensure. The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Cohesive regulatory framework for drugs. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology.
How to request FDA Device Classification 513(g) Alternative
Fda Frame Advanced Manufacturing Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. For example, it approved 2. The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Cohesive regulatory framework for drugs. Seek and analyze input to ensure.
From www.rikvin.com
Advanced Manufacturing in Singapore How is it Moving Ahead? Fda Frame Advanced Manufacturing The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. For example, it approved 2. Seek and analyze input to ensure. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai. Fda Frame Advanced Manufacturing.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Fda Frame Advanced Manufacturing Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: For example, it approved 2. The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Her keynote address focused on. Fda Frame Advanced Manufacturing.
From www.slideteam.net
Quarterly Roadmap Of Medical Device Approval From FDA Regulatory Fda Frame Advanced Manufacturing Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: For example, it approved 2. Cohesive regulatory framework for drugs. The food and drug administration (fda or agency) is announcing publication of a discussion. Fda Frame Advanced Manufacturing.
From www.biomarker.org
FDA Action Alert Sarepta, Protalix, Otsuka/Lundbeck and More Fda Frame Advanced Manufacturing Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. The food and drug administration (fda or agency) is announcing publication of a discussion. Fda Frame Advanced Manufacturing.
From greenstreet.net.au
How advanced manufacturing technologies can support more sustainable Fda Frame Advanced Manufacturing Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Cohesive regulatory framework for drugs. For example, it approved 2. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Her keynote address focused. Fda Frame Advanced Manufacturing.
From www.fda.gov
CDER’s Framework for Regulatory Advanced Manufacturing Evaluation Fda Frame Advanced Manufacturing For example, it approved 2. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Her keynote address focused on the role of ai. Fda Frame Advanced Manufacturing.
From formiventos.com
Advanced Manufacturing Technologies Designation Program Formiventos Fda Frame Advanced Manufacturing The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Fda defines “advanced manufacturing”. Fda Frame Advanced Manufacturing.
From www.researchgate.net
Priority ANDA FDA Review program under GDUFA II According the figure Fda Frame Advanced Manufacturing Cohesive regulatory framework for drugs. For example, it approved 2. Seek and analyze input to ensure. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees. Fda Frame Advanced Manufacturing.
From engine.roa.ai
Advanced Manufacturing Trends CB Insights 로아AI Fda Frame Advanced Manufacturing Cohesive regulatory framework for drugs. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information. Fda Frame Advanced Manufacturing.
From www.pnc.com
6 Trends to Know about in Manufacturing Technology PNC Insights Fda Frame Advanced Manufacturing Cohesive regulatory framework for drugs. For example, it approved 2. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Her. Fda Frame Advanced Manufacturing.
From medtechintelligence.com
Column Compliance Date Approaching for FDA Unique Device Identifiers Fda Frame Advanced Manufacturing Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. For example, it approved 2. Cohesive regulatory framework for drugs. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies:. Fda Frame Advanced Manufacturing.
From www.researchgate.net
The frame work for the developed additive manufacturing technology [20 Fda Frame Advanced Manufacturing Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. For example, it approved 2. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. The food. Fda Frame Advanced Manufacturing.
From www.aumanufacturing.com.au
Stay ready The basics of advanced manufacturing Australian Fda Frame Advanced Manufacturing Cohesive regulatory framework for drugs. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. For example, it approved 2. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages.. Fda Frame Advanced Manufacturing.
From avalere.com
FDA Approach to Oversight of COVID19 Therapeutics Has Evolved Avalere Fda Frame Advanced Manufacturing The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Cohesive regulatory framework for drugs. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Seek and analyze input to. Fda Frame Advanced Manufacturing.
From www.opans.com
OpAns Homepage Fda Frame Advanced Manufacturing The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Seek and analyze input to ensure. Cohesive regulatory framework for drugs. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information. Fda Frame Advanced Manufacturing.
From www.breakthroughvictoria.com
Advanced manufacturing Breakthrough Victoria Fda Frame Advanced Manufacturing The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. Cohesive regulatory framework for drugs. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Her keynote address focused on the. Fda Frame Advanced Manufacturing.
From endpts.com
How Patients with Epilepsy Benefit from RealWorld Data Endpoints News Fda Frame Advanced Manufacturing The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. Seek and analyze input to ensure. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Frame. Fda Frame Advanced Manufacturing.
From www.dreamstime.com
Advanced Manufacturing Icon. Monochrome Simple Sign from Digitalization Fda Frame Advanced Manufacturing Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Cohesive regulatory framework for drugs. Seek and analyze input to ensure. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way. Fda Frame Advanced Manufacturing.
From www.cellauto.com
解读 FDA药品审评和研究中心发布《药品生产中的人工智能》讨论稿 赛动智造 Fda Frame Advanced Manufacturing For example, it approved 2. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Cohesive regulatory framework for drugs. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is. Fda Frame Advanced Manufacturing.
From www.vrogue.co
The Medical Device Development Process The Red Outlin vrogue.co Fda Frame Advanced Manufacturing Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Cohesive regulatory framework for drugs. For example, it approved 2.. Fda Frame Advanced Manufacturing.
From eitanmedical.com
DRUG DELIVERY DEVICES THROUGHOUT THE DRUG DEVELOPMENT CYCLE Eitan Medical Fda Frame Advanced Manufacturing Seek and analyze input to ensure. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Cohesive regulatory framework for drugs. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: For example, it approved 2. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address. Fda Frame Advanced Manufacturing.
From dronavista.pl
Pavo30 Frame Kit (no carbon brace) Fda Frame Advanced Manufacturing Seek and analyze input to ensure. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Cohesive regulatory framework for. Fda Frame Advanced Manufacturing.
From capitolbh.com
Frame Manufacturing Capitol Builders Hardware Inc Fda Frame Advanced Manufacturing Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Cohesive regulatory framework for drugs. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: For example,. Fda Frame Advanced Manufacturing.
From www.advancedmanufacturingmadrid.com
Racks Series Advanced Manufacturing Madrid Fda Frame Advanced Manufacturing Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: For example, it approved 2. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Cohesive regulatory framework for drugs.. Fda Frame Advanced Manufacturing.
From www.manufacturingusa.com
National Strategy for Advanced Manufacturing Manufacturing USA Fda Frame Advanced Manufacturing For example, it approved 2. Cohesive regulatory framework for drugs. The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Frame focuses on four key priorities to develop a regulatory framework. Fda Frame Advanced Manufacturing.
From amta.sg
Advanced Manufacturing Lead AMLead AMTA Fda Frame Advanced Manufacturing Seek and analyze input to ensure. For example, it approved 2. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. The food and drug administration (fda or agency) is announcing publication of a discussion. Fda Frame Advanced Manufacturing.
From westchestermagazine.com
Advanced Manufacturing How to Get the Job Skills in Westchester Fda Frame Advanced Manufacturing The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Cohesive regulatory framework for drugs. Seek. Fda Frame Advanced Manufacturing.
From www.youtube.com
FDA Regulation of Medical Devices and Software/Apps YouTube Fda Frame Advanced Manufacturing The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. The food and drug administration (fda or agency). Fda Frame Advanced Manufacturing.
From www.rxtrace.com
FDA DSCSA Implementation Plan.Readable RxTrace Fda Frame Advanced Manufacturing Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Her keynote address focused on the role of ai in pharmaceutical. Fda Frame Advanced Manufacturing.
From dtamproject.eu
Bridging the Skills Gap Addressing the Challenges in Advanced Fda Frame Advanced Manufacturing Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Seek and analyze input to ensure. Cohesive regulatory framework for drugs. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: The food and drug administration (fda or agency) is announcing publication of a. Fda Frame Advanced Manufacturing.
From 3dprintingindustry.com
Researchers investigate bonding mechanisms during aluminum alloy 3D Fda Frame Advanced Manufacturing Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Cohesive regulatory framework for drugs. Seek and analyze input to ensure. The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. The food. Fda Frame Advanced Manufacturing.
From www.biomarker.org
FDA Approves Fabry Disease Treatment from Chiesi, Protalix Biomarker News Fda Frame Advanced Manufacturing Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Cohesive regulatory framework for drugs. The food and drug administration (fda or agency) is announcing publication of a discussion paper providing information for. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Her keynote address focused on the role of. Fda Frame Advanced Manufacturing.
From www.fda.gov
Accelerating the Adoption of Advanced Manufacturing Technologies to Fda Frame Advanced Manufacturing Cohesive regulatory framework for drugs. Her keynote address focused on the role of ai in pharmaceutical manufacturing and told attendees that ai is a priority technology. Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. The food and drug administration. Fda Frame Advanced Manufacturing.
From medicaldeviceacademy.com
How to request FDA Device Classification 513(g) Alternative Fda Frame Advanced Manufacturing Frame focuses on four key priorities to develop a regulatory framework for advanced manufacturing technologies: Seek and analyze input to ensure. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. For example, it approved 2. Cohesive regulatory framework. Fda Frame Advanced Manufacturing.
From www.biomarker.org
FDA Shows Confidence in Pfizer’s Maternal RSV Shot Ahead of Panel Fda Frame Advanced Manufacturing The food and drug administration has promoted advanced manufacturing—i.e., innovative technologies—as a way to address drug shortages. Fda defines “advanced manufacturing” in the amt draft guidance as an “innovative pharmaceutical manufacturing. Cohesive regulatory framework for drugs. For example, it approved 2. Seek and analyze input to ensure. The food and drug administration (fda or agency) is announcing publication of a. Fda Frame Advanced Manufacturing.