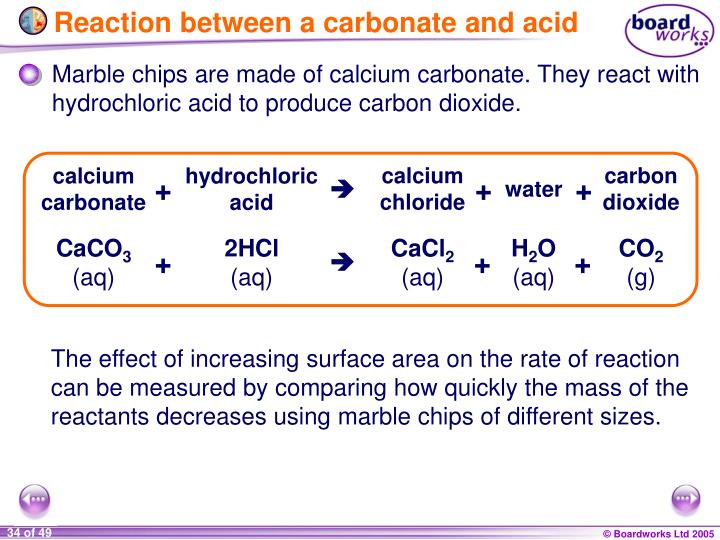
Magnesium Carbonate Reaction With Hydrochloric Acid . Magnesium carbonate reacts with hydrochloric acid to produce magnesium chloride, carbon dioxide, and water. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring Acid + metal → salt + hydrogen. Acid + metal → salt. The reaction is as follows. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Hydrochloric acid + magnesium →. The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch Acids react with metals to produce a salt and hydrogen. The equation for the reaction is: When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water.
from www.slideserve.com
The equation for the reaction is: One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Hydrochloric acid + magnesium →. When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Magnesium carbonate reacts with hydrochloric acid to produce magnesium chloride, carbon dioxide, and water. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring Acid + metal → salt + hydrogen. The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch Acid + metal → salt.
PPT IGCSE Chemistry PowerPoint Presentation ID5408111
Magnesium Carbonate Reaction With Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring The equation for the reaction is: Acid + metal → salt + hydrogen. Magnesium carbonate reacts with hydrochloric acid to produce magnesium chloride, carbon dioxide, and water. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Acids react with metals to produce a salt and hydrogen. When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. Hydrochloric acid + magnesium →. Acid + metal → salt. The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch The reaction is as follows.
From www.numerade.com
SOLVEDA 6.53 g sample of a mixture of magnesium carbonate and calcium Magnesium Carbonate Reaction With Hydrochloric Acid Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Acids will. Magnesium Carbonate Reaction With Hydrochloric Acid.
From sak-laop.blogspot.com
Magnesium And Hydrochloric Acid The rate of reaction of magnesium Magnesium Carbonate Reaction With Hydrochloric Acid The reaction is as follows. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.vrogue.co
Sodium Carbonate Reacts With Hydrochloric Acid Balanc vrogue.co Magnesium Carbonate Reaction With Hydrochloric Acid The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch The reaction is as follows. Acid + metal → salt. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. Hydrochloric acid + magnesium →. When hydrochloric. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.flickr.com
Reaction of Magnesium and Hydrochloric acid. Eric Schrader Flickr Magnesium Carbonate Reaction With Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. The equation for the reaction is: When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon. Magnesium Carbonate Reaction With Hydrochloric Acid.
From rowannewswest.blogspot.com
Balanced Equation of Sodium Carbonate and Hydrochloric Acid Magnesium Carbonate Reaction With Hydrochloric Acid One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. The reaction is as follows. Acid + metal → salt + hydrogen. Acids will react with reactive metals, such as. Magnesium Carbonate Reaction With Hydrochloric Acid.
From linativewise.blogspot.com
Calcium Carbonate and Hydrochloric Acid Magnesium Carbonate Reaction With Hydrochloric Acid Acid + metal → salt. The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch Acid + metal → salt + hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Magnesium carbonate reacts with. Magnesium Carbonate Reaction With Hydrochloric Acid.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Carbonate Reaction With Hydrochloric Acid Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring Acid + metal → salt + hydrogen. The equation for the reaction is: When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.youtube.com
Magnesium reacting with Hydrochloric Acid YouTube Magnesium Carbonate Reaction With Hydrochloric Acid Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring Magnesium carbonate reacts with hydrochloric acid to produce magnesium chloride, carbon dioxide, and water. Acid + metal → salt + hydrogen. Acid + metal → salt. The. Magnesium Carbonate Reaction With Hydrochloric Acid.
From mavink.com
Magnesium Hydrochloric Acid Magnesium Carbonate Reaction With Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acids react with metals to produce a salt and hydrogen. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation ID5408111 Magnesium Carbonate Reaction With Hydrochloric Acid One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring When hydrochloric. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.vrogue.co
Solved 9 Calcium Carbonate Reacts With Hydrochloric A vrogue.co Magnesium Carbonate Reaction With Hydrochloric Acid Magnesium carbonate reacts with hydrochloric acid to produce magnesium chloride, carbon dioxide, and water. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Acids react with metals to produce a salt and hydrogen. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) +. Magnesium Carbonate Reaction With Hydrochloric Acid.
From fineartamerica.com
Magnesium Reacting With Acid Photograph by Andrew Lambert Photography Magnesium Carbonate Reaction With Hydrochloric Acid The equation for the reaction is: Acids react with metals to produce a salt and hydrogen. The reaction is as follows. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.studocu.com
Lab 07 Reaction of Magnesium with Hydrochloric Acid (H2 Gen)1 The Magnesium Carbonate Reaction With Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The reaction is as follows. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Hydrochloric acid + magnesium →. Acid + metal → salt. When hydrochloric acid. Magnesium Carbonate Reaction With Hydrochloric Acid.
From mungfali.com
Magnesium Hydrochloric Acid Reaction Magnesium Carbonate Reaction With Hydrochloric Acid Acid + metal → salt. When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. The reaction is as follows. Hydrochloric acid + magnesium →. Acids react with metals to produce a salt and hydrogen. Magnesium carbonate reacts with hydrochloric acid to produce magnesium chloride, carbon dioxide, and water. One mole of magnesium carbonate [mgco. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.slideserve.com
PPT DO NOW!! PowerPoint Presentation, free download ID1560113 Magnesium Carbonate Reaction With Hydrochloric Acid Acids react with metals to produce a salt and hydrogen. The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid,. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.youtube.com
Magnesium & Hydrochloric Acid Reaction YouTube Magnesium Carbonate Reaction With Hydrochloric Acid Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring Hydrochloric acid + magnesium →. The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch The reaction is. Magnesium Carbonate Reaction With Hydrochloric Acid.
From complianceportal.american.edu
💋 Rate of reaction of magnesium with hydrochloric acid. Rate of Magnesium Carbonate Reaction With Hydrochloric Acid The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. Acids react with metals to produce a salt and hydrogen. Hydrochloric acid + magnesium →. The reaction is as follows. Magnesium + hydrochloric acid → magnesium. Magnesium Carbonate Reaction With Hydrochloric Acid.
From childhealthpolicy.vumc.org
😝 Reacting magnesium with hydrochloric acid. The Reactivity of Magnesium Carbonate Reaction With Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The reaction is as follows. The equation for the reaction is: Acid + metal → salt + hydrogen. When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. One mole of magnesium carbonate [mgco 3] and two moles. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.tessshebaylo.com
Chemical Equation For The Acid Ionization Of Hydrochloric Hcl In Water Magnesium Carbonate Reaction With Hydrochloric Acid The reaction is as follows. The equation for the reaction is: One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch Hydrochloric acid + magnesium →.. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science hive Magnesium Carbonate Reaction With Hydrochloric Acid Acid + metal → salt + hydrogen. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring The reaction is as follows. The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a. Magnesium Carbonate Reaction With Hydrochloric Acid.
From mammothmemory.net
Similarly magnesium and hydrochloric acid is a slow reaction Magnesium Carbonate Reaction With Hydrochloric Acid When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. The reaction is as follows. Acids react with metals to produce a salt and hydrogen. The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch Hydrochloric acid + magnesium →. Acids will react with reactive metals,. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.slideserve.com
PPT The reaction of magnesium oxide with dilute hydrochloric acid Magnesium Carbonate Reaction With Hydrochloric Acid When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. The reaction is as follows. Acid + metal → salt. Acids react with metals to produce a salt and hydrogen. Acid + metal → salt + hydrogen. The equation for the reaction is: Acids will react with reactive metals, such as magnesium and zinc, to. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.numerade.com
SOLVED A 6.53 g sample of a mixture of magnesium carbonate and Magnesium Carbonate Reaction With Hydrochloric Acid The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch Acid + metal → salt. When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium. Magnesium Carbonate Reaction With Hydrochloric Acid.
From pixels.com
Magnesium Carbonate In Hydrochloric Acid Photograph by Martyn F Magnesium Carbonate Reaction With Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Acid + metal → salt + hydrogen. Acid + metal → salt. When hydrochloric acid and magnesium carbonate. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.sciencephoto.com
Magnesium reacts with hydrochloric acid Stock Image C028/0732 Magnesium Carbonate Reaction With Hydrochloric Acid One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. The equation for the reaction is: Acid + metal → salt + hydrogen. When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. Magnesium + hydrochloric acid → magnesium chloride. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.chegg.com
Solved Magnesium metal reacts with hydrochloric acid to Magnesium Carbonate Reaction With Hydrochloric Acid When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. Acid + metal → salt + hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Hydrochloric acid + magnesium →. Magnesium carbonate reacts with hydrochloric acid to produce. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.youtube.com
Magnesium and Hydrochloric Acid Lab YouTube Magnesium Carbonate Reaction With Hydrochloric Acid One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. The equation for the reaction is: Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.slideserve.com
PPT Energy Change During Chemical Reactions PowerPoint Presentation Magnesium Carbonate Reaction With Hydrochloric Acid One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. Acid + metal → salt + hydrogen. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) →. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.alamy.com
Magnesium carbonate reacting with hydrochloric acid. This reaction Magnesium Carbonate Reaction With Hydrochloric Acid One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring Hydrochloric acid. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.youtube.com
What Happens when Magnesium reacts with Acetic Acid And Dilute Magnesium Carbonate Reaction With Hydrochloric Acid The reaction is as follows. The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch Acid + metal → salt + hydrogen. The equation for the reaction is: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Magnesium + hydrochloric acid → magnesium. Magnesium Carbonate Reaction With Hydrochloric Acid.
From igcsechemistryrevision.weebly.com
iGCSE CHEMISTRY REVISION HELP The Periodic Table Magnesium Carbonate Reaction With Hydrochloric Acid Acids react with metals to produce a salt and hydrogen. Acid + metal → salt + hydrogen. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring The reaction is as follows. Acid + metal → salt.. Magnesium Carbonate Reaction With Hydrochloric Acid.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Carbonate Reaction With Hydrochloric Acid One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch Acid + metal → salt. Acids react with metals to produce a salt and hydrogen. When. Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.nagwa.com
Question Video Writing a Net Ionic Equation for the Reaction of Solid Magnesium Carbonate Reaction With Hydrochloric Acid Hydrochloric acid + magnesium →. Acids react with metals to produce a salt and hydrogen. When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. Magnesium carbonate reacts with hydrochloric acid to produce magnesium chloride, carbon dioxide, and water. Magnesium + hydrochloric acid → magnesium chloride + hydrogen mg(s) + 2hcl(aq) → mgcl 2 (aq). Magnesium Carbonate Reaction With Hydrochloric Acid.
From www.scribd.com
Magnesium Reacts With Dilute Hydrochloric Acid in a Conical Flask Which Magnesium Carbonate Reaction With Hydrochloric Acid The rate of reaction between magnesium and dilute hydrochloric acid can be measured using a gas syringe and stopwatch When hydrochloric acid and magnesium carbonate react, they produce magnesium chloride, carbon dioxide, and water. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Hydrochloric. Magnesium Carbonate Reaction With Hydrochloric Acid.
From pixels.com
Magnesiumacid Reaction Photograph by Science Photo Library Pixels Magnesium Carbonate Reaction With Hydrochloric Acid One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Hydrochloric acid + magnesium →. The reaction is as follows. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. The. Magnesium Carbonate Reaction With Hydrochloric Acid.