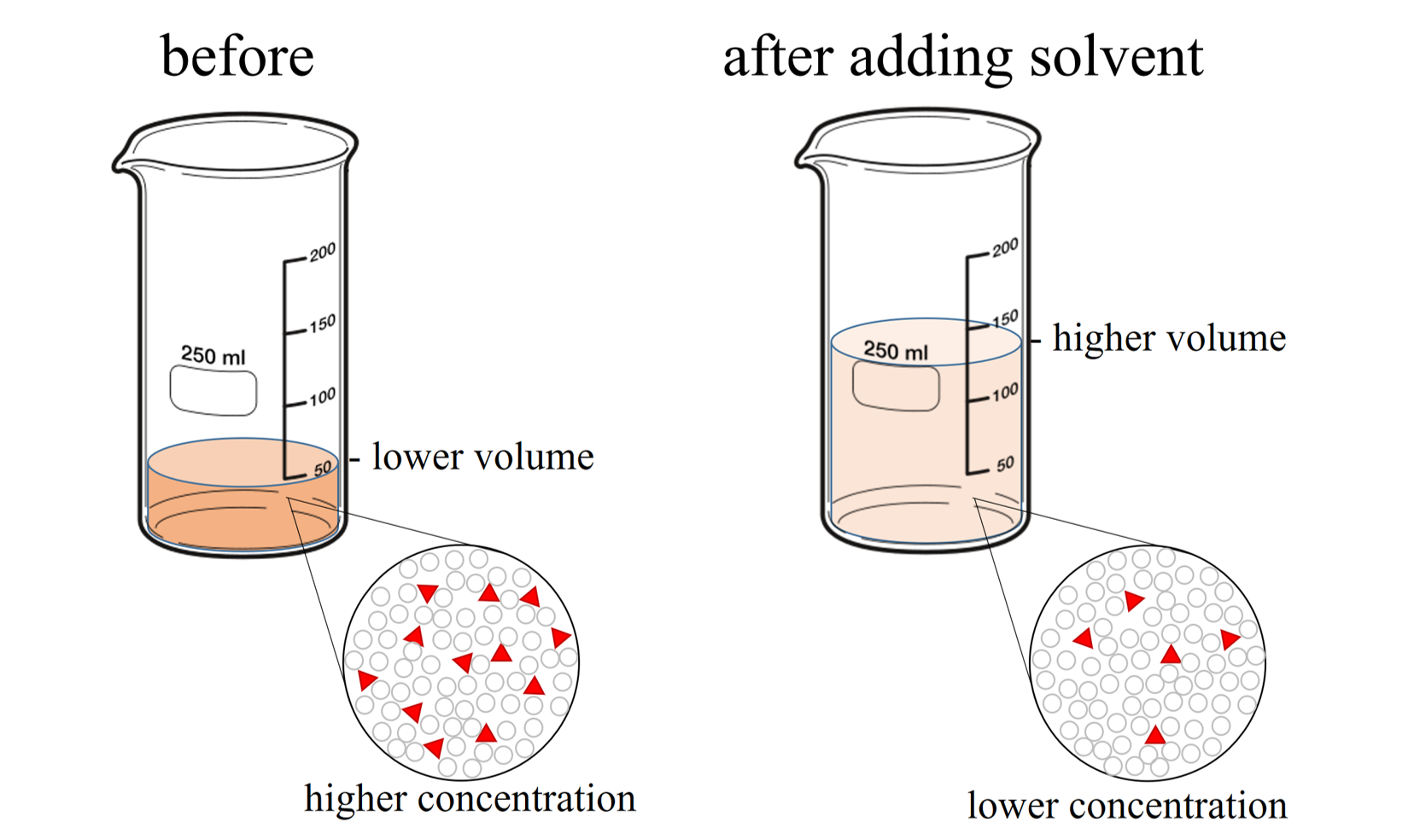
Dilution Chemical Composition . Concentration is the removal of. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration and dilutions of solutions | learning lab. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The dilution equation is a simple relation between concentrations and.
from chem.libretexts.org
Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. Concentration and dilutions of solutions | learning lab. Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of solvent, which increases the concentration of. The dilution equation is a simple relation between concentrations and. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution.
14.7 Solution Dilution Chemistry LibreTexts
Dilution Chemical Composition Concentration and dilutions of solutions | learning lab. Concentration is the removal of. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. The dilution equation is a simple relation between concentrations and. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration and dilutions of solutions | learning lab. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilution Chemical Composition The dilution equation is a simple relation between concentrations and. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. The solute concentration. Dilution Chemical Composition.
From www.pinterest.cl
Dilution when solvent is added to dilute a solution, the number of Dilution Chemical Composition Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. The dilution equation is a simple relation between concentrations and. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is. Dilution Chemical Composition.
From dalconhygiene.com.au
Download Chemical Dilution Chart Dalcon Hygiene Dilution Chemical Composition The dilution equation is a simple relation between concentrations and. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of. Dilution Chemical Composition.
From mavink.com
Chemical Dilution Chart Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration and dilutions of solutions | learning lab. Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in. Dilution Chemical Composition.
From spmchemistry.blog.onlinetuition.com.my
Dilution SPM Chemistry Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration and dilutions of solutions | learning lab. The dilution equation is a simple relation between concentrations and. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the. Dilution Chemical Composition.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution Chemical Composition Concentration and dilutions of solutions | learning lab. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of solvent, which increases the concentration. Dilution Chemical Composition.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Chemical Composition Concentration and dilutions of solutions | learning lab. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition. Dilution Chemical Composition.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Concentration is the removal of solvent, which increases the concentration of. The dilution equation is a simple relation between concentrations and. Dilution is the addition of. Dilution Chemical Composition.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration. Dilution Chemical Composition.
From dalconhygiene.com.au
Chemical Dilution Rate Guide Dalcon Hygiene Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent,. Dilution Chemical Composition.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. The solute concentration of a solution may be decreased by adding solvent, a. Dilution Chemical Composition.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration and dilutions of solutions | learning lab. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition. Dilution Chemical Composition.
From www.researchgate.net
(PDF) A New Method for Weld Dilution Calculation through Chemical Dilution Chemical Composition Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. \ [\require {mhchem}\] concentration refers to. Dilution Chemical Composition.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration and dilutions of solutions | learning lab. Concentration is the removal of. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. Dilution Chemical Composition.
From centuryclever846.weebly.com
Bacterial Serial Dilution Method centuryclever Dilution Chemical Composition \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute. Dilution Chemical Composition.
From pressurewashingresource.com
Chem dilution chart 2 by Chemical/Chemistry Dilution Chemical Composition Concentration is the removal of solvent, which increases the concentration of. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a simple relation between concentrations and. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of. Dilution Chemical Composition.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Dilution Chemical Composition Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of solvent, which increases the concentration of. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal. Dilution Chemical Composition.
From studylib.net
Dilution Ratios Table Dilution Chemical Composition Concentration and dilutions of solutions | learning lab. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. \ [\require {mhchem}\] concentration. Dilution Chemical Composition.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Chemical Composition The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of. Dilution Chemical Composition.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. The solute concentration of a solution. Dilution Chemical Composition.
From www.youtube.com
Dilution Lab Results Chemistry Matters YouTube Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration and dilutions of solutions | learning lab. Concentration is the removal of. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The dilution equation is. Dilution Chemical Composition.
From www.youtube.com
How To Dilute Chemicals Dilution Ratios Explained! YouTube Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration and dilutions of solutions | learning lab. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which. Dilution Chemical Composition.
From www.thoughtco.com
Dilution Calculations From Stock Solutions in Chemistry Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Dilution is the addition of solvent, which decreases the concentration. Dilution Chemical Composition.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The dilution equation is a simple relation between concentrations and. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration and. Dilution Chemical Composition.
From studylib.net
Chemical dilution chart. Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. Concentration is the removal of solvent, which increases the concentration of. The dilution equation is a simple relation between concentrations and. Concentration is the removal of. Dilution is the. Dilution Chemical Composition.
From www.youtube.com
Ce qu'il faut savoir sur la dilution en chimie (Cours 2de) YouTube Dilution Chemical Composition Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. Dilution is the addition of solvent, which decreases the concentration of. Dilution Chemical Composition.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID Dilution Chemical Composition The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent,. Dilution Chemical Composition.
From www.researchgate.net
As Welded Chemical Composition and Dilution Factor Download Dilution Chemical Composition Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Concentration and dilutions of solutions | learning lab. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. \ [\require. Dilution Chemical Composition.
From pressurewashingresource.com
Chem dilution chart 4 by JayDavis Chemical/Chemistry Pressure Dilution Chemical Composition \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. Concentration and dilutions of solutions | learning lab. Concentration is the removal of solvent, which increases the concentration of. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Concentration is the removal of solvent, which increases. Dilution Chemical Composition.
From www.youtube.com
Serial Dilution Technique For Microbiological & Chemical Analysis Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration and dilutions of solutions | learning lab. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. Concentration is the removal of solvent, which increases the concentration of. The dilution equation is a simple relation between concentrations and.. Dilution Chemical Composition.
From www.researchgate.net
Schematic representation of the dilution system. Download Scientific Dilution Chemical Composition Concentration is the removal of solvent, which increases the concentration of. Concentration and dilutions of solutions | learning lab. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the. Dilution Chemical Composition.
From www.actioncleanup.com
Dilution Action’s Guide to Mixing the Right Solutions Dilution Chemical Composition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The dilution equation is a simple relation between concentrations and. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration and dilutions of solutions | learning. Dilution Chemical Composition.
From www.bics.org.uk
A3 Dilution Chart BICSc Dilution Chemical Composition Concentration and dilutions of solutions | learning lab. The dilution equation is a simple relation between concentrations and. Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition. Dilution Chemical Composition.
From www.pinterest.com.mx
Example of a Serial Dilution Medical laboratory science, Medical Dilution Chemical Composition Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a simple relation between concentrations and. Dilution is the addition of solvent, which decreases the concentration of. Dilution Chemical Composition.
From www.sliderbase.com
Strengths of Acids and Bases Making Dilutions Presentation Chemistry Dilution Chemical Composition Concentration is the removal of. \ [\require {mhchem}\] concentration refers to the amount of solute present in a given quantity. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. The dilution equation is a simple relation between concentrations and. Dilution is the. Dilution Chemical Composition.