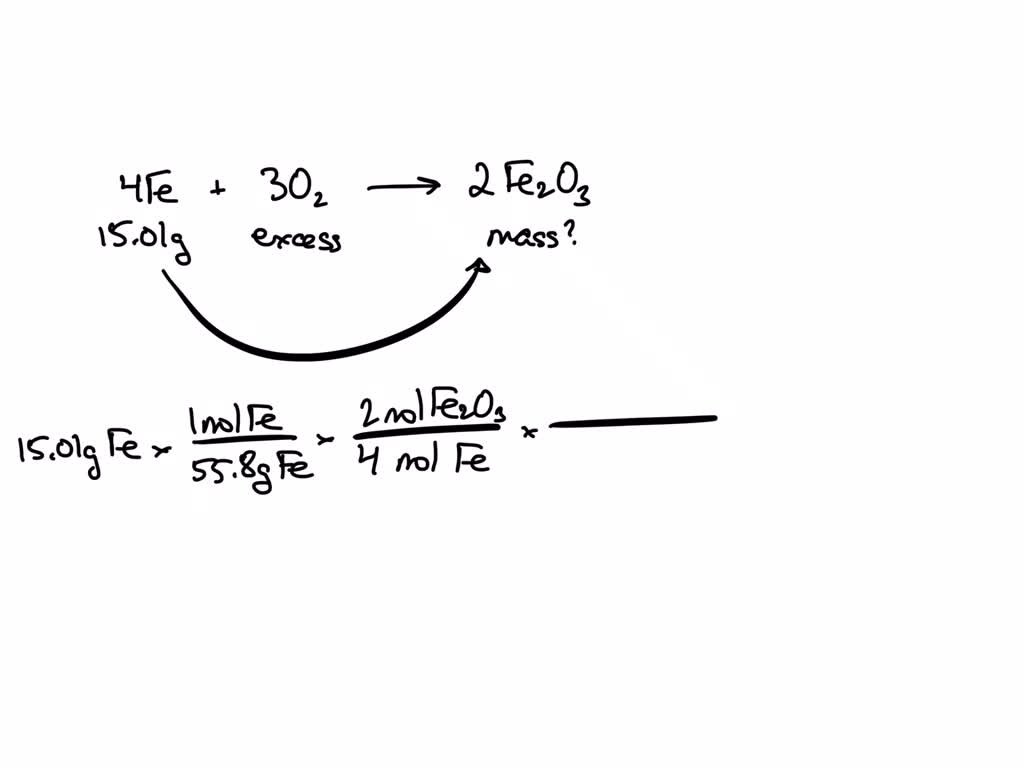
Iron (Iii) Oxide And Water Formula . Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. 4 f e + 3 o 2 → 2 f e 2 o 3. Ferric oxide, also called by its iupac name iron trihydrate or iron (iii) oxide, is an inorganic compound represented by the. The oxidation state of fe 2 o 3. Oxidation is the process in which an atom loses an electron. Iron (iii) oxide has the formula fe 2 o 3. 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. The chemical equation for this reaction is: It is a strong oxidizing agent and reacts with. It is a black solid that is insoluble in water. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. Iron (iii) oxide has the chemical formula f e 2 o 3. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. It is formed when iron undergoes oxidation.
from www.numerade.com
Oxidation is the process in which an atom loses an electron. Iron (iii) oxide has the formula fe 2 o 3. It is formed when iron undergoes oxidation. Iron (iii) oxide has the chemical formula f e 2 o 3. It is a black solid that is insoluble in water. The chemical equation for this reaction is: Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Ferric oxide, also called by its iupac name iron trihydrate or iron (iii) oxide, is an inorganic compound represented by the. In this video we will describe the equation fe2o3 + h2o and write what happens when fe2o3 is. It is a strong oxidizing agent and reacts with.
SOLVED Iron reacts with oxygen to form iron(III) oxide according to
Iron (Iii) Oxide And Water Formula The chemical equation for this reaction is: It is formed when iron undergoes oxidation. The chemical equation for this reaction is: Iron (iii) oxide has the chemical formula f e 2 o 3. It is a black solid that is insoluble in water. Iron (iii) oxide has the formula fe 2 o 3. 4 f e + 3 o 2 → 2 f e 2 o 3. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. It is a strong oxidizing agent and reacts with. The oxidation state of fe 2 o 3. 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. In this video we will describe the equation fe2o3 + h2o and write what happens when fe2o3 is. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Oxidation is the process in which an atom loses an electron. Ferric oxide, also called by its iupac name iron trihydrate or iron (iii) oxide, is an inorganic compound represented by the.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Iron (Iii) Oxide And Water Formula Oxidation is the process in which an atom loses an electron. 4 f e + 3 o 2 → 2 f e 2 o 3. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. The oxidation state of fe 2 o 3. In this video we will describe the equation fe2o3 + h2o. Iron (Iii) Oxide And Water Formula.
From www.youtube.com
Equation for Fe(NO3)3 + H2O Iron (III) nitrate + Water YouTube Iron (Iii) Oxide And Water Formula The chemical equation for this reaction is: Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Oxidation is the process in which an atom loses an electron. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms.. Iron (Iii) Oxide And Water Formula.
From www.numerade.com
SOLVEDThe formation of rust [Iron (III) oxide] on the surface of iron Iron (Iii) Oxide And Water Formula Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. The chemical equation for this reaction is: It is a strong oxidizing agent and reacts with. Oxidation is the process in which an atom loses an electron. Both methods result in the formation of iron (iii) oxide, commonly used. Iron (Iii) Oxide And Water Formula.
From testbook.com
Iron(III) Oxide Formula Know Its Preparation, Properties & Uses Iron (Iii) Oxide And Water Formula When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. The oxidation state of fe 2 o 3. 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. Fe 2 o 3 is the chemical formula of iron (iii) oxide which. Iron (Iii) Oxide And Water Formula.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Iron (Iii) Oxide And Water Formula When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. It is a strong oxidizing agent and reacts with. In this video we will describe the equation fe2o3 + h2o and write what happens when fe2o3 is. Iron (iii) oxide has the formula fe 2 o 3. Oxidation is the process in which an. Iron (Iii) Oxide And Water Formula.
From www.numerade.com
SOLVED The thermite reaction involves aluminum and iron(III) oxide Iron (Iii) Oxide And Water Formula The chemical equation for this reaction is: Ferric oxide, also called by its iupac name iron trihydrate or iron (iii) oxide, is an inorganic compound represented by the. 4 f e + 3 o 2 → 2 f e 2 o 3. It is a strong oxidizing agent and reacts with. Iron (iii) oxide has the formula fe 2 o. Iron (Iii) Oxide And Water Formula.
From www.numerade.com
SOLVED The thermal of iron(III) hydroxide to produce Iron (Iii) Oxide And Water Formula When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry.. Iron (Iii) Oxide And Water Formula.
From www.chegg.com
Solved The chemical equation below represents the reaction Iron (Iii) Oxide And Water Formula It is a strong oxidizing agent and reacts with. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. The oxidation state of fe 2 o 3. Iron (iii) oxide has the formula fe 2 o 3. It is formed when iron undergoes oxidation. 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated. Iron (Iii) Oxide And Water Formula.
From www.slideserve.com
PPT Stoichiometry with a Twist PowerPoint Presentation ID1836706 Iron (Iii) Oxide And Water Formula Iron (iii) oxide has the chemical formula f e 2 o 3. Iron (iii) oxide has the formula fe 2 o 3. It is formed when iron undergoes oxidation. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Ferric oxide, also called by its iupac name iron trihydrate or iron (iii) oxide, is. Iron (Iii) Oxide And Water Formula.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) Redox Iron (Iii) Oxide And Water Formula 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Ferric oxide, also called by its iupac name iron trihydrate or iron (iii) oxide, is an inorganic compound represented by the. 4 f e + 3 o 2 → 2 f e 2 o 3. The chemical equation for this reaction is: 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii). Iron (Iii) Oxide And Water Formula.
From www.youtube.com
How to Write the Formula for Iron (III) Oxide YouTube Iron (Iii) Oxide And Water Formula 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. It is a strong oxidizing agent and reacts with. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Fe 2 o 3 is the. Iron (Iii) Oxide And Water Formula.
From www.youtube.com
How to write the formula for iron (III) oxide YouTube Iron (Iii) Oxide And Water Formula 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. It is formed when iron undergoes oxidation. Ferric oxide, also called by its iupac name iron trihydrate or iron (iii) oxide, is an inorganic compound represented by the. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Iron (iii) oxide. Iron (Iii) Oxide And Water Formula.
From www.toppr.com
Iron III Oxide Formula Definition, Concepts and Examples Iron (Iii) Oxide And Water Formula 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. Iron (iii) oxide has the chemical formula f e 2. Iron (Iii) Oxide And Water Formula.
From www.numerade.com
SOLVED Calculate the mass of Iron(III) oxide (FezO;) that contains a Iron (Iii) Oxide And Water Formula In this video we will describe the equation fe2o3 + h2o and write what happens when fe2o3 is. 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. Iron (iii) oxide has the chemical formula f e 2 o 3. Ferric oxide, also called by its iupac. Iron (Iii) Oxide And Water Formula.
From joigjogsq.blob.core.windows.net
Iron Iii Oxide Negative Ion at Emily Gaines blog Iron (Iii) Oxide And Water Formula 4 f e + 3 o 2 → 2 f e 2 o 3. Iron (iii) oxide has the formula fe 2 o 3. It is a black solid that is insoluble in water. It is a strong oxidizing agent and reacts with. It is formed when iron undergoes oxidation. When you heat iron (iii) hydroxide, it breaks down to. Iron (Iii) Oxide And Water Formula.
From www.slideserve.com
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379 Iron (Iii) Oxide And Water Formula In this video we will describe the equation fe2o3 + h2o and write what happens when fe2o3 is. It is a strong oxidizing agent and reacts with. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments. Iron (Iii) Oxide And Water Formula.
From www.slideserve.com
PPT IB DP1 Chemistry Bonding PowerPoint Presentation, free download Iron (Iii) Oxide And Water Formula 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. Iron (iii) oxide has the chemical formula f e 2 o 3. Oxidation is the process in which an atom loses an electron. In this video we will describe the equation fe2o3 + h2o and write what. Iron (Iii) Oxide And Water Formula.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate Iron (Iii) Oxide And Water Formula Ferric oxide, also called by its iupac name iron trihydrate or iron (iii) oxide, is an inorganic compound represented by the. Oxidation is the process in which an atom loses an electron. Iron (iii) oxide has the chemical formula f e 2 o 3. It is a strong oxidizing agent and reacts with. The chemical equation for this reaction is:. Iron (Iii) Oxide And Water Formula.
From www.slideserve.com
PPT Unit Chemical Reactions PowerPoint Presentation, free download Iron (Iii) Oxide And Water Formula The oxidation state of fe 2 o 3. It is a black solid that is insoluble in water. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. The chemical equation for this reaction is: 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron. Iron (Iii) Oxide And Water Formula.
From www.bartleby.com
Answered Iron and water react to form iron(III)… bartleby Iron (Iii) Oxide And Water Formula 4 f e + 3 o 2 → 2 f e 2 o 3. 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. The oxidation state of fe 2 o 3. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and. Iron (Iii) Oxide And Water Formula.
From www.sciencephoto.com
Iron (III) oxide in water and in hydrochloric acid Stock Image C036 Iron (Iii) Oxide And Water Formula It is formed when iron undergoes oxidation. 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. It is a black solid that is insoluble in water. Both methods result in. Iron (Iii) Oxide And Water Formula.
From www.pw.live
Iron III Oxide Formula, Structure, Properties, Uses Iron (Iii) Oxide And Water Formula Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. The chemical equation for this reaction is: 4 f e + 3 o 2 → 2 f e 2 o 3. Oxidation is the process in which an atom loses an electron. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. When. Iron (Iii) Oxide And Water Formula.
From www.showme.com
Iron III oxide Science, Chemistry, Chemical Bonds ShowMe Iron (Iii) Oxide And Water Formula Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. The chemical equation for this reaction is: It is a black solid that is insoluble in. Iron (Iii) Oxide And Water Formula.
From www.numerade.com
SOLVED of iron with oxygen gas and water produces iron (III) oxide (a Iron (Iii) Oxide And Water Formula The chemical equation for this reaction is: Iron (iii) oxide has the formula fe 2 o 3. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. It is a strong oxidizing agent and reacts with. It is a black solid that is insoluble in water. Oxidation is the. Iron (Iii) Oxide And Water Formula.
From www.chegg.com
Solved Iron and water react to form iron(III) oxide and Iron (Iii) Oxide And Water Formula 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. It is formed when iron undergoes oxidation. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. It is a strong oxidizing agent and reacts with. Oxidation is the process in which an atom loses an electron. 4 f e +. Iron (Iii) Oxide And Water Formula.
From joifprggj.blob.core.windows.net
Iron (Iii) Oxide Boiling Point at Mark High blog Iron (Iii) Oxide And Water Formula It is a strong oxidizing agent and reacts with. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. The oxidation state of fe 2 o 3. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. It is formed when iron undergoes. Iron (Iii) Oxide And Water Formula.
From www.numerade.com
SOLVED Iron reacts with oxygen to form iron(III) oxide according to Iron (Iii) Oxide And Water Formula Iron (iii) oxide has the chemical formula f e 2 o 3. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Oxidation is the process in which an atom loses an electron. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms.. Iron (Iii) Oxide And Water Formula.
From www.youtube.com
Gas stoichiometry of iron(III) hydroxide YouTube Iron (Iii) Oxide And Water Formula The chemical equation for this reaction is: It is a strong oxidizing agent and reacts with. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. The oxidation state of fe 2 o 3. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when. Iron (Iii) Oxide And Water Formula.
From stock.adobe.com
iron III Oxide Properties and Chemical Compound Structure Stock Vector Iron (Iii) Oxide And Water Formula Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. In this video we will describe the equation fe2o3 + h2o and write what happens when fe2o3 is. It is a strong oxidizing agent and reacts with. Ferric oxide, also called by its. Iron (Iii) Oxide And Water Formula.
From www.chegg.com
Solved 6. Iron reacts with oxygen gas to form iron (III) Iron (Iii) Oxide And Water Formula The chemical equation for this reaction is: Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. When you heat iron (iii) hydroxide, it breaks down to form. Iron (Iii) Oxide And Water Formula.
From www.youtube.com
What is the formula for iron III oxide? YouTube Iron (Iii) Oxide And Water Formula When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. It is formed when iron undergoes oxidation. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Oxidation is the process in which an atom loses an. Iron (Iii) Oxide And Water Formula.
From www.slideserve.com
PPT Combustion analysis PowerPoint Presentation, free download ID Iron (Iii) Oxide And Water Formula In this video we will describe the equation fe2o3 + h2o and write what happens when fe2o3 is. It is a black solid that is insoluble in water. Ferric oxide, also called by its iupac name iron trihydrate or iron (iii) oxide, is an inorganic compound represented by the. Iron (iii) oxide has the formula fe 2 o 3. The. Iron (Iii) Oxide And Water Formula.
From www.youtube.com
How to Balance Fe + O2 + H2O = Fe2O3 . xH2O (Iron + Oxygen gas + Water Iron (Iii) Oxide And Water Formula When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms.. Iron (Iii) Oxide And Water Formula.
From www.chegg.com
Solved Iron and water react to form iron(III) oxide and Iron (Iii) Oxide And Water Formula 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. 4 f e + 3 o 2 → 2 f e 2 o 3. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. 2𝐹𝑒 (𝑂𝐻)₃. Iron (Iii) Oxide And Water Formula.
From www.sciencephoto.com
Iron (III) oxide in water Stock Image C036/3131 Science Photo Library Iron (Iii) Oxide And Water Formula 4 f e + 3 o 2 → 2 f e 2 o 3. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. 4fe + 3o2 + 2h2o → 4feo (oh) the hydrated iron (iii) oxide when dehydrated at 200°c produces iron (iii) oxide alongside the. It is formed when iron undergoes oxidation.. Iron (Iii) Oxide And Water Formula.