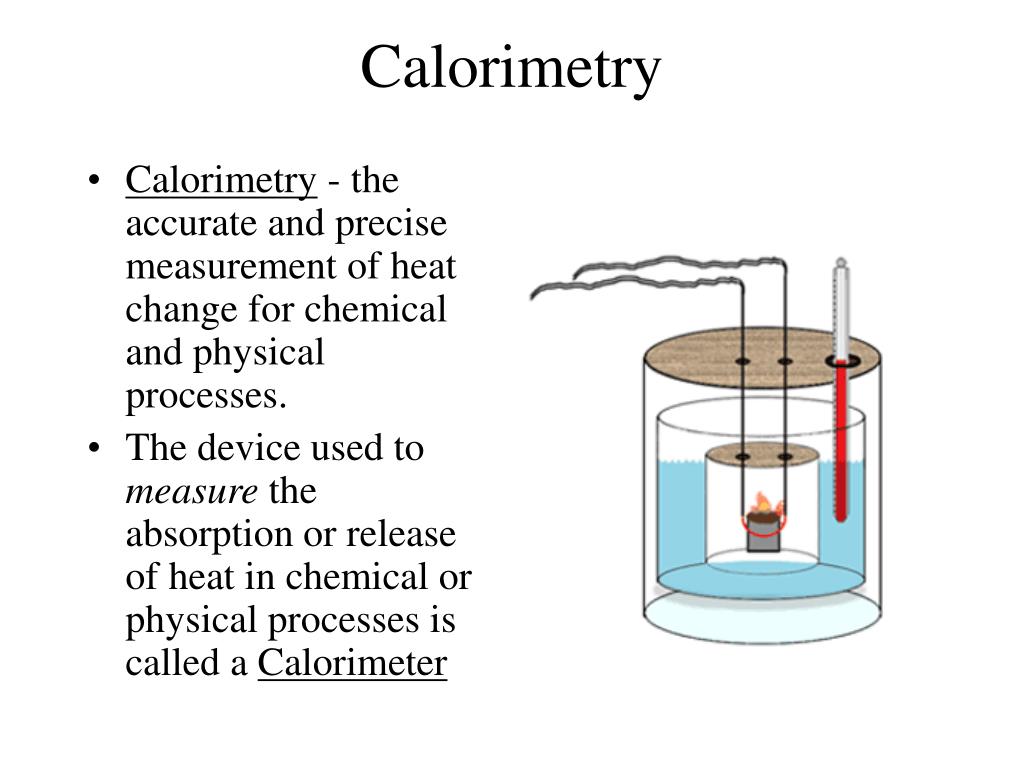
Why Is Calorimetry Important In Chemistry . Find out the types of calorimeters, the calorimetry equation,. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. calorimetry belongs to the most important experimental techniques. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It is the only experimental method allowing for. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change.
from www.slideserve.com
calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It is the only experimental method allowing for. Find out the types of calorimeters, the calorimetry equation,. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. calorimetry belongs to the most important experimental techniques. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change.
PPT Chapter 11 PowerPoint Presentation, free download ID1084959
Why Is Calorimetry Important In Chemistry learn how calorimetry measures the amount of heat involved in physical or chemical reactions. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Find out the types of calorimeters, the calorimetry equation,. calorimetry belongs to the most important experimental techniques. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It is the only experimental method allowing for.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Why Is Calorimetry Important In Chemistry learn how calorimetry measures the amount of heat involved in physical or chemical reactions. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Find out the types of calorimeters, the calorimetry equation,. a calorimeter is a device used to measure the amount of heat involved in a. Why Is Calorimetry Important In Chemistry.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Why Is Calorimetry Important In Chemistry Find out the types of calorimeters, the calorimetry equation,. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry belongs to the most important experimental techniques. If a reaction between two substances shall be started. Why Is Calorimetry Important In Chemistry.
From www.thoughtco.com
What is Calorimetry in Chemistry? Why Is Calorimetry Important In Chemistry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. It is the only. Why Is Calorimetry Important In Chemistry.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Why Is Calorimetry Important In Chemistry calorimetry belongs to the most important experimental techniques. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. a calorimeter is a device used to measure the amount of heat involved in. Why Is Calorimetry Important In Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Why Is Calorimetry Important In Chemistry calorimetry belongs to the most important experimental techniques. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Why Is Calorimetry Important In Chemistry.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation Why Is Calorimetry Important In Chemistry It is the only experimental method allowing for. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. Find out the types of calorimeters, the calorimetry equation,. calorimetry belongs to the most important experimental techniques. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Why Is Calorimetry Important In Chemistry.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Why Is Calorimetry Important In Chemistry Find out the types of calorimeters, the calorimetry equation,. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry belongs to the most important experimental techniques. calorimetry. Why Is Calorimetry Important In Chemistry.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry Important In Chemistry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. calorimetry belongs to the most important experimental techniques.. Why Is Calorimetry Important In Chemistry.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Why Is Calorimetry Important In Chemistry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry belongs to the most important experimental techniques. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. calorimetry is the process of measuring the amount of heat released or absorbed during a. Why Is Calorimetry Important In Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Why Is Calorimetry Important In Chemistry learn how calorimetry measures the amount of heat involved in physical or chemical reactions. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. calorimetry measures enthalpy changes during chemical processes, where. Why Is Calorimetry Important In Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID9539914 Why Is Calorimetry Important In Chemistry It is the only experimental method allowing for. calorimetry belongs to the most important experimental techniques. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. calorimetry is the process of measuring the amount of heat released. Why Is Calorimetry Important In Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Why Is Calorimetry Important In Chemistry It is the only experimental method allowing for. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Find out the types of calorimeters, the calorimetry equation,. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. learn how. Why Is Calorimetry Important In Chemistry.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Why Is Calorimetry Important In Chemistry If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It is the only experimental method. Why Is Calorimetry Important In Chemistry.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Why Is Calorimetry Important In Chemistry Find out the types of calorimeters, the calorimetry equation,. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. learn how calorimetry measures the amount of heat involved in physical or chemical reactions.. Why Is Calorimetry Important In Chemistry.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Why Is Calorimetry Important In Chemistry calorimetry belongs to the most important experimental techniques. It is the only experimental method allowing for. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. calorimetry is the process of measuring the amount of heat released or. Why Is Calorimetry Important In Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Why Is Calorimetry Important In Chemistry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry belongs to the most important experimental techniques. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. Why Is Calorimetry Important In Chemistry.
From saylordotorg.github.io
Calorimetry Why Is Calorimetry Important In Chemistry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Find out the types of calorimeters, the calorimetry equation,. calorimetry is the process of measuring the amount of heat released or absorbed during a. Why Is Calorimetry Important In Chemistry.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Why Is Calorimetry Important In Chemistry If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. Find out the types of calorimeters, the calorimetry equation,. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry belongs to the most important experimental techniques. It is the only experimental method allowing for.. Why Is Calorimetry Important In Chemistry.
From www.thoughtco.com
Calorimeter Definition in Chemistry Why Is Calorimetry Important In Chemistry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. calorimetry is the process. Why Is Calorimetry Important In Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Why Is Calorimetry Important In Chemistry calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. It is the only experimental method allowing for. calorimetry. Why Is Calorimetry Important In Chemistry.
From testbook.com
Principle of Calorimetry Definition, Formula, Uses, Examples Why Is Calorimetry Important In Chemistry learn how calorimetry measures the amount of heat involved in physical or chemical reactions. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the. Why Is Calorimetry Important In Chemistry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Why Is Calorimetry Important In Chemistry calorimetry belongs to the most important experimental techniques. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a. Why Is Calorimetry Important In Chemistry.
From studylib.net
Calorimetry Why Is Calorimetry Important In Chemistry If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Find out the types. Why Is Calorimetry Important In Chemistry.
From www.youtube.com
6.2 part2 AP Chemistry Constant volume calorimetry YouTube Why Is Calorimetry Important In Chemistry learn how calorimetry measures the amount of heat involved in physical or chemical reactions. calorimetry belongs to the most important experimental techniques. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Why Is Calorimetry Important In Chemistry.
From www.pinterest.com
Calorimetry Why Is Calorimetry Important In Chemistry calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Find out the types of calorimeters, the calorimetry equation,. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. It. Why Is Calorimetry Important In Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Why Is Calorimetry Important In Chemistry learn how calorimetry measures the amount of heat involved in physical or chemical reactions. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved. Why Is Calorimetry Important In Chemistry.
From ecampusontario.pressbooks.pub
5.2 Calorimetry Chemistry Why Is Calorimetry Important In Chemistry calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry belongs to the most important experimental techniques. a calorimeter is a device used to measure the amount of heat involved in a. Why Is Calorimetry Important In Chemistry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID5405762 Why Is Calorimetry Important In Chemistry calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Find out the types of calorimeters, the calorimetry equation,. calorimetry belongs to the most important experimental techniques. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. calorimetry is the process of measuring the amount of heat. Why Is Calorimetry Important In Chemistry.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Why Is Calorimetry Important In Chemistry learn how calorimetry measures the amount of heat involved in physical or chemical reactions. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Find out the types of calorimeters, the calorimetry equation,. It. Why Is Calorimetry Important In Chemistry.
From studylib.net
Calorimetry Why Is Calorimetry Important In Chemistry calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry belongs to the most important experimental techniques. Find out the types of calorimeters, the calorimetry equation,. a calorimeter is. Why Is Calorimetry Important In Chemistry.
From www.youtube.com
Principle of Calorimetry YouTube Why Is Calorimetry Important In Chemistry calorimetry belongs to the most important experimental techniques. Find out the types of calorimeters, the calorimetry equation,. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. It is the only experimental method allowing for. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is.. Why Is Calorimetry Important In Chemistry.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Why Is Calorimetry Important In Chemistry calorimetry belongs to the most important experimental techniques. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a. Why Is Calorimetry Important In Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Why Is Calorimetry Important In Chemistry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry belongs to the most important experimental techniques. It is the only experimental method allowing for. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. calorimetry is the process of measuring the. Why Is Calorimetry Important In Chemistry.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Why Is Calorimetry Important In Chemistry calorimetry belongs to the most important experimental techniques. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Find out. Why Is Calorimetry Important In Chemistry.
From www.youtube.com
Calorimetry (AQA A level Chemistry) YouTube Why Is Calorimetry Important In Chemistry Find out the types of calorimeters, the calorimetry equation,. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. learn how calorimetry measures the amount of heat involved in physical or chemical reactions. If a reaction between two substances shall be started or accelerated, the chemist’s first choice is.. Why Is Calorimetry Important In Chemistry.