Does Salt Dissolve In Methanol . Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. the solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and methanol + ethanol as well as those of sodium bromide in water +. if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different. Thus, we expect it to be soluble in water. Because of the oh group in methanol, we expect its molecules to be polar. furthermore salt solubilities in the mixed solvents (water + methanol, water + ethanol, water + acetone, methanol + ethanol, methanol + acetone, ethanol + acetone) were determined at 313.15 k. july 13, 2023 by techiescience core sme. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. because water is polar, substances that are polar or ionic will dissolve in it. organic compounds tend to dissolve well in solvents that have similar. This is because the water is able.
from www.slideserve.com
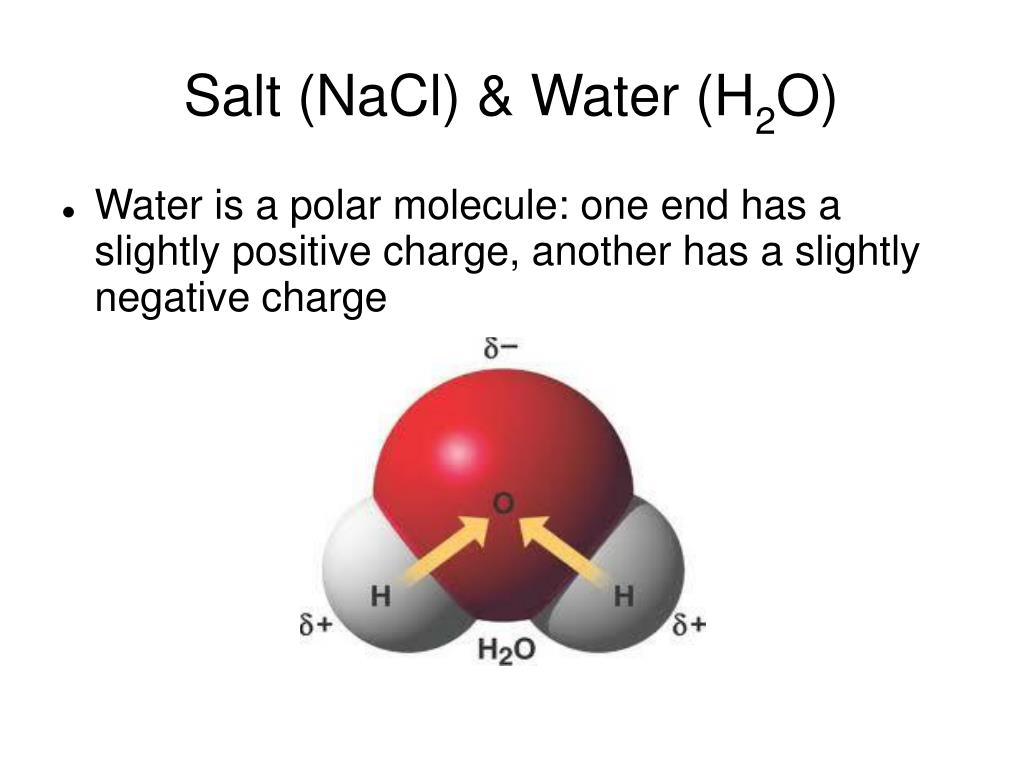
because water is polar, substances that are polar or ionic will dissolve in it. Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. Thus, we expect it to be soluble in water. july 13, 2023 by techiescience core sme. the solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and methanol + ethanol as well as those of sodium bromide in water +. organic compounds tend to dissolve well in solvents that have similar. furthermore salt solubilities in the mixed solvents (water + methanol, water + ethanol, water + acetone, methanol + ethanol, methanol + acetone, ethanol + acetone) were determined at 313.15 k. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different. This is because the water is able.
PPT Intermolecular Forces PowerPoint Presentation, free download ID
Does Salt Dissolve In Methanol july 13, 2023 by techiescience core sme. if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. This is because the water is able. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. furthermore salt solubilities in the mixed solvents (water + methanol, water + ethanol, water + acetone, methanol + ethanol, methanol + acetone, ethanol + acetone) were determined at 313.15 k. Because of the oh group in methanol, we expect its molecules to be polar. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different. july 13, 2023 by techiescience core sme. organic compounds tend to dissolve well in solvents that have similar. because water is polar, substances that are polar or ionic will dissolve in it. Thus, we expect it to be soluble in water. the solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and methanol + ethanol as well as those of sodium bromide in water +.
From www.ozmo.io
Methanol Its Uses Properties And Effects Does Salt Dissolve In Methanol In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different. if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. july 13, 2023 by techiescience core sme.. Does Salt Dissolve In Methanol.
From mungfali.com
Dissolving Salt In Water Diagram Does Salt Dissolve In Methanol This is because the water is able. if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. july 13, 2023 by techiescience core sme. organic compounds tend to dissolve well in solvents. Does Salt Dissolve In Methanol.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Does Salt Dissolve In Methanol because water is polar, substances that are polar or ionic will dissolve in it. Thus, we expect it to be soluble in water. july 13, 2023 by techiescience core sme. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. In table 1, the measured solubilities of the salts in water, methanol,. Does Salt Dissolve In Methanol.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Does Salt Dissolve In Methanol The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. organic compounds tend to dissolve well in solvents that have similar. because water is polar, substances that are polar or ionic will dissolve in it. Because of the oh group in methanol, we expect its molecules to be polar. the solubilities. Does Salt Dissolve In Methanol.
From saylordotorg.github.io
Physical Properties of Alcohols Does Salt Dissolve In Methanol The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. Because of the oh group in methanol, we expect its molecules to be polar. furthermore salt solubilities in the mixed solvents (water + methanol, water + ethanol, water + acetone, methanol + ethanol, methanol + acetone, ethanol + acetone) were determined at 313.15. Does Salt Dissolve In Methanol.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Does Salt Dissolve In Methanol july 13, 2023 by techiescience core sme. organic compounds tend to dissolve well in solvents that have similar. Because of the oh group in methanol, we expect its molecules to be polar. Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. Thus, we expect it to be soluble in water. the solubilities. Does Salt Dissolve In Methanol.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Does Salt Dissolve In Methanol furthermore salt solubilities in the mixed solvents (water + methanol, water + ethanol, water + acetone, methanol + ethanol, methanol + acetone, ethanol + acetone) were determined at 313.15 k. july 13, 2023 by techiescience core sme. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different. Because of the oh group. Does Salt Dissolve In Methanol.
From www.ozmo.io
How Does Water Dissolve Most Salts? Does Salt Dissolve In Methanol Thus, we expect it to be soluble in water. Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. Because of the oh group in methanol, we expect its molecules to be polar. the solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and methanol + ethanol as well. Does Salt Dissolve In Methanol.
From pubs.acs.org
Solubility of Electrolytes in Organic Solvents SolventSpecific Does Salt Dissolve In Methanol Thus, we expect it to be soluble in water. if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically. Does Salt Dissolve In Methanol.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Does Salt Dissolve In Methanol organic compounds tend to dissolve well in solvents that have similar. the solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and methanol + ethanol as well as those of sodium bromide in water +. Thus, we expect it to be soluble in water. In table 1, the measured solubilities of the. Does Salt Dissolve In Methanol.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Does Salt Dissolve In Methanol Because of the oh group in methanol, we expect its molecules to be polar. This is because the water is able. the solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and methanol + ethanol as well as those of sodium bromide in water +. In table 1, the measured solubilities of the. Does Salt Dissolve In Methanol.
From diydistilling.com
What are the symptoms of methanol poisoning (And how to treat it Does Salt Dissolve In Methanol furthermore salt solubilities in the mixed solvents (water + methanol, water + ethanol, water + acetone, methanol + ethanol, methanol + acetone, ethanol + acetone) were determined at 313.15 k. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. In table 1, the measured solubilities of the salts in water, methanol, and. Does Salt Dissolve In Methanol.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Does Salt Dissolve In Methanol Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. This is because the water is able. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. july 13, 2023 by techiescience core sme. Thus, we expect it to be soluble in water. furthermore salt solubilities in the. Does Salt Dissolve In Methanol.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Does Salt Dissolve In Methanol furthermore salt solubilities in the mixed solvents (water + methanol, water + ethanol, water + acetone, methanol + ethanol, methanol + acetone, ethanol + acetone) were determined at 313.15 k. Thus, we expect it to be soluble in water. the solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and methanol +. Does Salt Dissolve In Methanol.
From www.researchgate.net
Chloroformmethanol precipitation. ( a ) 1.5 mL tube containing 500 μL Does Salt Dissolve In Methanol The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. furthermore salt solubilities in the mixed solvents (water + methanol, water + ethanol, water + acetone, methanol + ethanol, methanol + acetone, ethanol + acetone) were determined at 313.15 k. In table 1, the measured solubilities of the salts in water, methanol, and. Does Salt Dissolve In Methanol.
From youmemindbody.com
The Facts About Methamphetamine Addiction YouMeMindBody Does Salt Dissolve In Methanol This is because the water is able. if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. Because of the oh group in methanol, we expect its molecules to be polar. because water. Does Salt Dissolve In Methanol.
From www.semanticscholar.org
[PDF] Solubility of NaCl, NaBr, and KCl in Water, Methanol, Ethanol Does Salt Dissolve In Methanol This is because the water is able. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different. if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. . Does Salt Dissolve In Methanol.
From www.ozmo.io
When Salt Is Added To Water The Salt Dissolves And The Water Molecules Does Salt Dissolve In Methanol This is because the water is able. Thus, we expect it to be soluble in water. july 13, 2023 by techiescience core sme. organic compounds tend to dissolve well in solvents that have similar. Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. In table 1, the measured solubilities of the salts in. Does Salt Dissolve In Methanol.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Does Salt Dissolve In Methanol Thus, we expect it to be soluble in water. Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. because water is polar, substances that are polar or ionic will dissolve in it. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. the solubilities of sodium chloride,. Does Salt Dissolve In Methanol.
From www.youtube.com
Why Salt (NaCl) Does Not Dissolve in Oil Salt and Oil Experiment Is Does Salt Dissolve In Methanol Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. july 13, 2023 by techiescience core sme. Because of the oh group in methanol, we expect its molecules to be polar. This is because the water is able. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different. . Does Salt Dissolve In Methanol.
From courses.lumenlearning.com
Compounds Essential to Human Functioning Anatomy and Does Salt Dissolve In Methanol In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different. if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. organic compounds tend to dissolve well in. Does Salt Dissolve In Methanol.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Does Salt Dissolve In Methanol organic compounds tend to dissolve well in solvents that have similar. because water is polar, substances that are polar or ionic will dissolve in it. july 13, 2023 by techiescience core sme. Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. if we make a mixture of water, methanol and a. Does Salt Dissolve In Methanol.
From www.islandphysics.com
A Salty Sea Island Physics Does Salt Dissolve In Methanol the solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and methanol + ethanol as well as those of sodium bromide in water +. Because of the oh group in methanol, we expect its molecules to be polar. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically. Does Salt Dissolve In Methanol.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Does Salt Dissolve In Methanol if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. organic compounds tend to dissolve well in. Does Salt Dissolve In Methanol.
From www.youtube.com
Why Salt Dissolve In Water/உப்பு ஏன் தண்ணீரில் கரைகிறது YouTube Does Salt Dissolve In Methanol The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. furthermore salt solubilities in the mixed solvents (water + methanol, water + ethanol, water + acetone, methanol + ethanol, methanol + acetone, ethanol + acetone) were determined at 313.15 k. Methanol is a colorless liquid that is commonly used as a solvent, fuel,. Does Salt Dissolve In Methanol.
From fermentationskultur.blogspot.com
Capsaicinoide bei der Fermentationsdestillation Does Salt Dissolve In Methanol organic compounds tend to dissolve well in solvents that have similar. Because of the oh group in methanol, we expect its molecules to be polar. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different. if. Does Salt Dissolve In Methanol.
From yesdirt.com
Does Salt Dissolve In Alcohol? (ANSWERED) Yes Dirt Does Salt Dissolve In Methanol if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. Thus, we expect it to be soluble in water. the solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water,. Does Salt Dissolve In Methanol.
From www.slideserve.com
PPT Macromolecules PowerPoint Presentation, free download ID5948789 Does Salt Dissolve In Methanol july 13, 2023 by techiescience core sme. the solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and methanol + ethanol as well as those of sodium bromide in water +. Thus, we expect it to be soluble in water. The solubility of methanol refers to its ability to dissolve in a. Does Salt Dissolve In Methanol.
From www.americasrehabcampuses.com
Methamphetamine Abuse What are the side effects America's Rehab Campuses Does Salt Dissolve In Methanol if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. organic compounds tend to dissolve well in. Does Salt Dissolve In Methanol.
From rdsoberliving.com
How Long is Meth in My System? Real Deal Meth Info Does Salt Dissolve In Methanol because water is polar, substances that are polar or ionic will dissolve in it. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. the solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and methanol + ethanol as well as those of sodium bromide in. Does Salt Dissolve In Methanol.
From flatworldknowledge.lardbucket.org
Solubility and Molecular Structure Does Salt Dissolve In Methanol Thus, we expect it to be soluble in water. organic compounds tend to dissolve well in solvents that have similar. Because of the oh group in methanol, we expect its molecules to be polar. Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. In table 1, the measured solubilities of the salts in water,. Does Salt Dissolve In Methanol.
From www.numerade.com
SOLVEDAs the salt KNO3 dissolves in methanol, the number x(t) of grams Does Salt Dissolve In Methanol if we make a mixture of water, methanol and a salt like $\ce{fecl2}$, $\ce{nacl}$ etc, will the ability of water and methanol to mix allow $\ce{fecl2}$ and $\ce{nacl}$ to be a part of this mixture. Because of the oh group in methanol, we expect its molecules to be polar. The solubility of methanol refers to its ability to dissolve. Does Salt Dissolve In Methanol.
From www.researchgate.net
Solubility curve of IDA in methanol, ethanol, acetonitrile, acetone and Does Salt Dissolve In Methanol furthermore salt solubilities in the mixed solvents (water + methanol, water + ethanol, water + acetone, methanol + ethanol, methanol + acetone, ethanol + acetone) were determined at 313.15 k. Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. organic compounds tend to dissolve well in solvents that have similar. july 13,. Does Salt Dissolve In Methanol.
From builderbold.com
Does salt dissolve in alcohol? BuilderBold Does Salt Dissolve In Methanol furthermore salt solubilities in the mixed solvents (water + methanol, water + ethanol, water + acetone, methanol + ethanol, methanol + acetone, ethanol + acetone) were determined at 313.15 k. organic compounds tend to dissolve well in solvents that have similar. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. Because. Does Salt Dissolve In Methanol.
From dissolvesalt.weebly.com
scientific report does salt dissolve quicker in hot or cold water? Does Salt Dissolve In Methanol In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different. The solubility of methanol refers to its ability to dissolve in a particular solvent, typically water. Methanol is a colorless liquid that is commonly used as a solvent, fuel, and. because water is polar, substances that are polar or ionic will dissolve in. Does Salt Dissolve In Methanol.