What Ph Is 14 . In other words, it tells us how basic or acidic the solution is. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. A lower ph means something is. For example, concentrated hydrochloric acid. But the scale does not have fixed limits, so it is indeed possible to have a ph above 14 or below zero. The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. The ph scale measures whether there is more hydronium or hydroxide in a solution. Ph is a measure of how acidic or basic a substance is.
from stock.adobe.com
A lower ph means something is. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph is a measure of how acidic or basic a substance is. In other words, it tells us how basic or acidic the solution is. But the scale does not have fixed limits, so it is indeed possible to have a ph above 14 or below zero. The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. For example, concentrated hydrochloric acid.
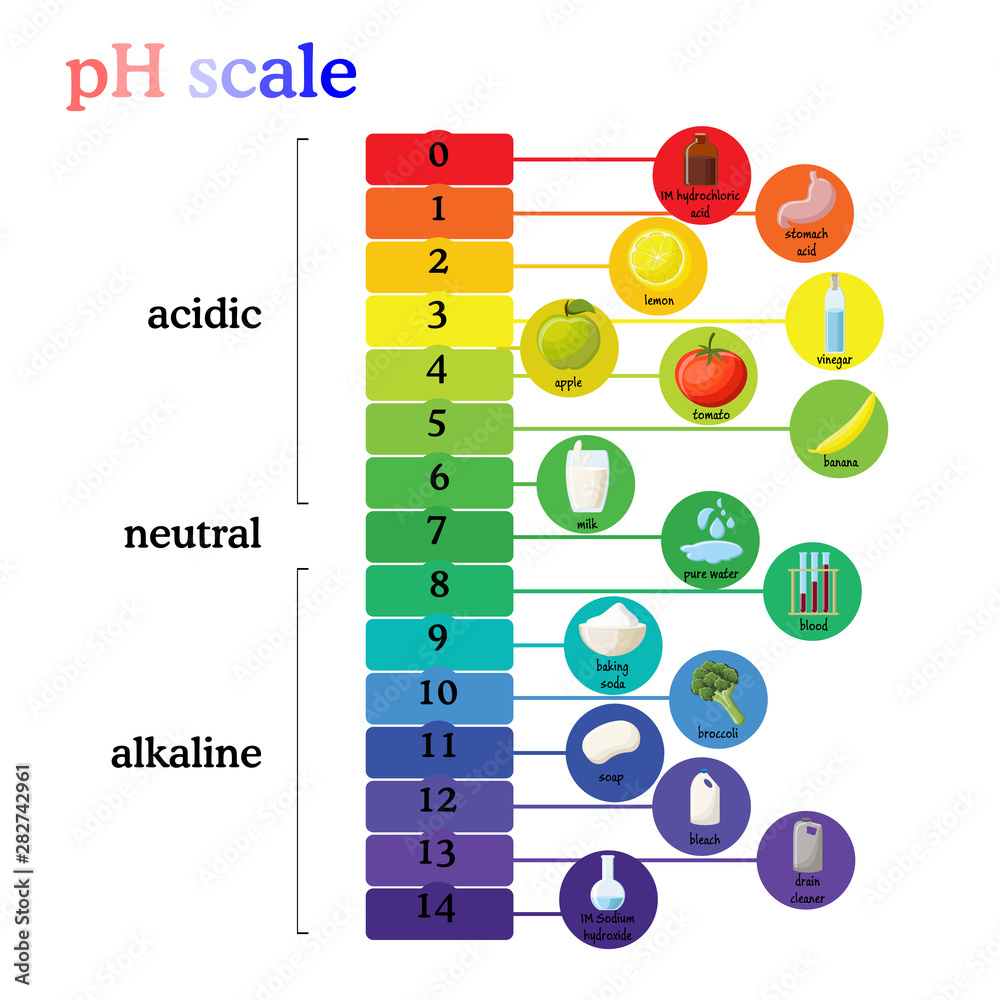
pH scale diagram with corresponding acidic or alkaline values for
What Ph Is 14 For example, concentrated hydrochloric acid. For example, concentrated hydrochloric acid. Ph is a measure of how acidic or basic a substance is. The ph scale measures whether there is more hydronium or hydroxide in a solution. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. In other words, it tells us how basic or acidic the solution is. A lower ph means something is. The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. But the scale does not have fixed limits, so it is indeed possible to have a ph above 14 or below zero. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the.
From www.newagenutrients.com
pH Scale newagenutrients What Ph Is 14 In other words, it tells us how basic or acidic the solution is. The ph scale measures whether there is more hydronium or hydroxide in a solution. Ph is a measure of how acidic or basic a substance is. But the scale does not have fixed limits, so it is indeed possible to have a ph above 14 or below. What Ph Is 14.
From lanamarconi.wordpress.com
ph scale What Ph Is 14 The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Ph is a measure of how acidic or basic a substance is. In other words, it tells us how basic or. What Ph Is 14.
From es.dreamstime.com
Escala Del Valor De PH Para Las Soluciones ácidas Y Alcalinas What Ph Is 14 It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. The ph scale measures whether there is more hydronium or hydroxide in a solution. A lower ph means something is. The ph range does not have an upper nor lower bound, since as defined above, the ph is. What Ph Is 14.
From acidsandbasesrios.weebly.com
pH Acids and Bases What Ph Is 14 The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. But the scale does not have fixed limits, so it is indeed possible to have a ph above 14 or. What Ph Is 14.
From www.alamy.com
The pH scale Universal Indicator pH Color Chart diagram acidic Stock What Ph Is 14 The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. For example, concentrated hydrochloric acid. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. Ph, quantitative measure of the acidity or. What Ph Is 14.
From sites.google.com
Mixtures AC MetzgarScience What Ph Is 14 The ph scale measures whether there is more hydronium or hydroxide in a solution. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. In other words, it tells us how basic or acidic the solution is. A lower ph means something is. The ph scale measures how strongly acidic or alkaline a. What Ph Is 14.
From www.freepik.com
Premium Vector Chart ph acidic, neutral and alkaline scale. Ph value What Ph Is 14 Ph is a measure of how acidic or basic a substance is. For example, concentrated hydrochloric acid. The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and. What Ph Is 14.
From www.dreamstime.com
The PH Scale Universal Indicator PH Color Chart Diagram Stock Vector What Ph Is 14 But the scale does not have fixed limits, so it is indeed possible to have a ph above 14 or below zero. Ph is a measure of how acidic or basic a substance is. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. It takes a numerical value between 0 and 14 that measures the. What Ph Is 14.
From www.mometrix.com
pH Overview (Chemistry Review Video) What Ph Is 14 Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The ph scale measures whether there is more hydronium or hydroxide in a solution. A lower ph means something is. In other words, it tells us how basic or acidic the solution is. For example, concentrated hydrochloric acid. The ph scale measures how strongly acidic or. What Ph Is 14.
From getwellstaywellathome.com
Using pH as a Health Monitor Get Well Stay Well At Home What Ph Is 14 Ph is a measure of how acidic or basic a substance is. The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. For example, concentrated hydrochloric acid. The ph scale measures. What Ph Is 14.
From www.alamy.com
The pH scale of common chemicals illustration Stock Vector Image & Art What Ph Is 14 Ph is a measure of how acidic or basic a substance is. A lower ph means something is. In other words, it tells us how basic or acidic the solution is. For example, concentrated hydrochloric acid. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. It takes a numerical value between 0. What Ph Is 14.
From topglobenews.com
What is the pH Scale? Top Globe News What Ph Is 14 The ph scale measures whether there is more hydronium or hydroxide in a solution. The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in. What Ph Is 14.
From sciencenotes.org
The pH Scale of Common Chemicals What Ph Is 14 The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. The ph scale measures whether there is more hydronium or hydroxide in a solution. For example, concentrated hydrochloric acid. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The ph scale measures how strongly acidic or alkaline a. What Ph Is 14.
From www.bigstockphoto.com
PH Scale Infographic Image & Photo (Free Trial) Bigstock What Ph Is 14 Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The ph scale measures whether there is more hydronium or hydroxide in a solution. Ph is a measure of how acidic or basic a substance is. A lower ph means something is. But the scale does not have fixed limits, so it is indeed possible to. What Ph Is 14.
From stock.adobe.com
PH Acid Scale Vector Illustration Diagram with Acidic, Neutral and What Ph Is 14 The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. A lower ph means something is. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. The. What Ph Is 14.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision What Ph Is 14 Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. For example, concentrated hydrochloric acid. But the scale does not have fixed limits, so it is indeed possible to have a ph above 14 or below zero. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. The ph. What Ph Is 14.
From www.youtube.com
⚠️ ¿Qué es el pH? Escala de pH, EJEMPLOS ⚠️ [Fácil y Rápido] QUÍMICA What Ph Is 14 A lower ph means something is. But the scale does not have fixed limits, so it is indeed possible to have a ph above 14 or below zero. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. The term, widely used in chemistry, biology, and agronomy,. What Ph Is 14.
From www.sliderbase.com
PH Calculations Presentation Chemistry What Ph Is 14 The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. A lower ph means something is. The ph scale measures whether there is more hydronium or hydroxide in a solution. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The. What Ph Is 14.
From www.alamy.es
La escala de pH sobre fondo blanco Imagen Vector de stock Alamy What Ph Is 14 A lower ph means something is. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. The term, widely used in chemistry, biology,. What Ph Is 14.
From www.toppr.com
pH Definition, Formula, Meaning, Applications and More What Ph Is 14 A lower ph means something is. For example, concentrated hydrochloric acid. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. The ph scale measures whether there is more hydronium. What Ph Is 14.
From mungfali.com
Ph Strip Color Chart What Ph Is 14 In other words, it tells us how basic or acidic the solution is. The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. The ph scale measures whether there is more hydronium or hydroxide in a solution. It takes a numerical value between 0 and. What Ph Is 14.
From byjus.com
Why pH is from 1 to 14? What Ph Is 14 The ph scale measures whether there is more hydronium or hydroxide in a solution. A lower ph means something is. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. For example, concentrated hydrochloric acid. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and. What Ph Is 14.
From naturalbiohealth.com
How Learning the pH Scale Can Create a More Balanced Diet Natural Bio What Ph Is 14 The ph scale measures whether there is more hydronium or hydroxide in a solution. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. A lower ph means something is. But the scale does not have fixed limits, so it is indeed possible to have a ph above. What Ph Is 14.
From stock.adobe.com
pH scale diagram with corresponding acidic or alkaline values for What Ph Is 14 Ph is a measure of how acidic or basic a substance is. In other words, it tells us how basic or acidic the solution is. For example, concentrated hydrochloric acid. But the scale does not have fixed limits, so it is indeed possible to have a ph above 14 or below zero. The ph scale measures how strongly acidic or. What Ph Is 14.
From japaneseclass.jp
Images of PHスポール JapaneseClass.jp What Ph Is 14 It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. In other words, it tells us how basic or acidic the solution is. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. For example, concentrated hydrochloric acid. A lower ph means something is.. What Ph Is 14.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG What Ph Is 14 The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. For example, concentrated hydrochloric acid. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The ph. What Ph Is 14.
From www.dreamstime.com
PH Scale. Universal Indicator PH Stock Vector Illustration of healthy What Ph Is 14 A lower ph means something is. The ph scale measures whether there is more hydronium or hydroxide in a solution. For example, concentrated hydrochloric acid. Ph is a measure of how acidic or basic a substance is. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14.. What Ph Is 14.
From mavink.com
Ph Colour Chart With Colour Names What Ph Is 14 The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. But the scale does not have fixed limits, so it is indeed possible to have a ph above 14 or. What Ph Is 14.
From themestizamuse.com
Understanding pH Balance and Hair — The Mestiza Muse What Ph Is 14 The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. In other words, it tells us how basic or acidic the solution is. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. But the. What Ph Is 14.
From chemwiki.ucdavis.edu
The pH Scale Chemwiki What Ph Is 14 Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in. What Ph Is 14.
From blog.juicegrape.com
how to test for pH Archives Musto Wine Grape Company, LLC What Ph Is 14 The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. But the scale does not have fixed limits, so it is indeed possible to have a ph above. What Ph Is 14.
From printable.rjuuc.edu.np
Printable Ph Scale What Ph Is 14 But the scale does not have fixed limits, so it is indeed possible to have a ph above 14 or below zero. The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. Ph is a measure of how acidic or basic a substance is. It. What Ph Is 14.
From www.alamy.com
Diagram of the pH scale with examples of acidic, neutral and alkaline What Ph Is 14 But the scale does not have fixed limits, so it is indeed possible to have a ph above 14 or below zero. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. Ph is a measure of how acidic or basic a substance is. Ph, quantitative measure of the acidity or basicity of. What Ph Is 14.
From www.sciencelearn.org.nz
pH scale — Science Learning Hub What Ph Is 14 Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. The ph range does not have an upper nor lower bound, since as defined above, the ph is an indication of concentration of h +. In other words, it. What Ph Is 14.
From www.dreamstime.com
The PH Scale Universal Indicator PH Color Chart Diagram Stock Vector What Ph Is 14 The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. In other words, it tells us how basic or acidic the solution is. The ph range does not have an. What Ph Is 14.