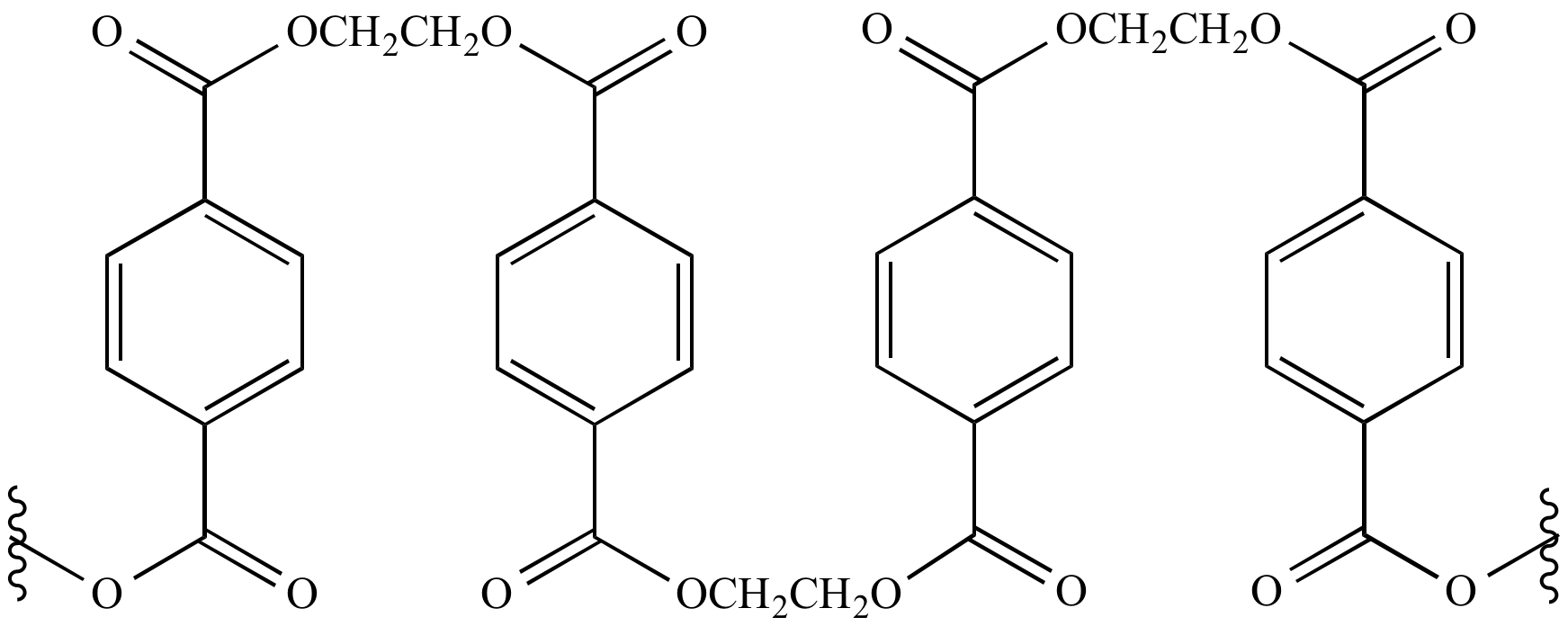
How Is Pet Made Chemistry . Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. Ethylene glycol is a colourless liquid. Polyethylene terephthalate (pet) is a macromolecule called a polyester. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. In order to synthesize pet the monomer of pet must be. To produce pet, chemists use two different kinds of monomers: Terephthalic acid and ethylene glycol. Pet / pete can also be recycled back to its original elements as well as into polyester fibres. Pet is produced by the polymerization of ethylene glycol and terephthalic acid.
from www.chem.ucla.edu
Terephthalic acid and ethylene glycol. Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. Polyethylene terephthalate (pet) is a macromolecule called a polyester. In order to synthesize pet the monomer of pet must be. Pet / pete can also be recycled back to its original elements as well as into polyester fibres. To produce pet, chemists use two different kinds of monomers: Ethylene glycol is a colourless liquid. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. Pet is produced by the polymerization of ethylene glycol and terephthalic acid.
Illustrated Glossary of Organic Chemistry Polyethylene terephthalate
How Is Pet Made Chemistry In order to synthesize pet the monomer of pet must be. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. Ethylene glycol is a colourless liquid. Pet / pete can also be recycled back to its original elements as well as into polyester fibres. Pet is produced by the polymerization of ethylene glycol and terephthalic acid. To produce pet, chemists use two different kinds of monomers: Terephthalic acid and ethylene glycol. Polyethylene terephthalate (pet) is a macromolecule called a polyester. In order to synthesize pet the monomer of pet must be. Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water.
From glossary.periodni.com
Polyester Chemistry Dictionary & Glossary How Is Pet Made Chemistry Ethylene glycol is a colourless liquid. Terephthalic acid and ethylene glycol. In order to synthesize pet the monomer of pet must be. To produce pet, chemists use two different kinds of monomers: It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. Pet. How Is Pet Made Chemistry.
From read.nxtbook.com
Plastics It’s All About Molecular Structure How Is Pet Made Chemistry Polyethylene terephthalate (pet) is a macromolecule called a polyester. Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. To produce pet, chemists use two different kinds of monomers: In order to synthesize pet the monomer of pet must be. Ethylene glycol is a colourless liquid. Terephthalic acid and. How Is Pet Made Chemistry.
From www.researchgate.net
Process of producing recycled polyester fibers from RPET Download How Is Pet Made Chemistry Terephthalic acid and ethylene glycol. To produce pet, chemists use two different kinds of monomers: It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. Pet is produced by the polymerization of ethylene glycol and terephthalic acid. In order to synthesize pet the. How Is Pet Made Chemistry.
From www.researchgate.net
Chemical reactions of PET manufacturing. Download Scientific Diagram How Is Pet Made Chemistry Terephthalic acid and ethylene glycol. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. Ethylene glycol is a colourless liquid. Pet. How Is Pet Made Chemistry.
From www.mdpi.com
IJMS Free FullText An Overview into Polyethylene Terephthalate How Is Pet Made Chemistry Pet is produced by the polymerization of ethylene glycol and terephthalic acid. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. Pet / pete can also be recycled back to its original elements as well as into polyester fibres. Both units undergo a reaction called esterification, where an. How Is Pet Made Chemistry.
From www.animalia-life.club
Pet Plastic Structure How Is Pet Made Chemistry Ethylene glycol is a colourless liquid. To produce pet, chemists use two different kinds of monomers: Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. Polyethylene terephthalate (pet) is a macromolecule called a polyester. Pet is produced by the polymerization of ethylene glycol and terephthalic acid. It is. How Is Pet Made Chemistry.
From www.researchgate.net
Products formed from the degradation of PET using PETases and previous How Is Pet Made Chemistry Pet is produced by the polymerization of ethylene glycol and terephthalic acid. To produce pet, chemists use two different kinds of monomers: Terephthalic acid and ethylene glycol. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. Pet / pete can also be recycled back to its original elements. How Is Pet Made Chemistry.
From www.acs.org
Poly(ethylene terephthalate) American Chemical Society How Is Pet Made Chemistry In order to synthesize pet the monomer of pet must be. Pet / pete can also be recycled back to its original elements as well as into polyester fibres. To produce pet, chemists use two different kinds of monomers: Polyethylene terephthalate (pet) is a macromolecule called a polyester. Ethylene glycol is a colourless liquid. It is a recyclable thermoplastic polymer. How Is Pet Made Chemistry.
From chem.libretexts.org
2.7 Synthesis of Esters Chemistry LibreTexts How Is Pet Made Chemistry To produce pet, chemists use two different kinds of monomers: Pet is produced by the polymerization of ethylene glycol and terephthalic acid. Ethylene glycol is a colourless liquid. Polyethylene terephthalate (pet) is a macromolecule called a polyester. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. Terephthalic acid. How Is Pet Made Chemistry.
From www.chemistrystudent.com
Addtion Polymerisation (ALevel) ChemistryStudent How Is Pet Made Chemistry Ethylene glycol is a colourless liquid. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. To produce pet, chemists use two different kinds of monomers: The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule. How Is Pet Made Chemistry.
From www.alamy.com
Polyethylene terephthalate or PET, PETE polyester, thermoplastic How Is Pet Made Chemistry Polyethylene terephthalate (pet) is a macromolecule called a polyester. Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. In order to synthesize pet the monomer of pet must be. Terephthalic acid and ethylene glycol. To produce pet, chemists use two different kinds of monomers: Pet is produced by. How Is Pet Made Chemistry.
From www.alamy.com
Polyethylene terephthalate or PET, PETE polyester, thermoplastic How Is Pet Made Chemistry Pet / pete can also be recycled back to its original elements as well as into polyester fibres. Ethylene glycol is a colourless liquid. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. Pet is produced by the polymerization of ethylene glycol and terephthalic acid. Both units undergo. How Is Pet Made Chemistry.
From www.nagwa.com
Question Video Identifying the Structure of Poly(Ethylene How Is Pet Made Chemistry Polyethylene terephthalate (pet) is a macromolecule called a polyester. Pet / pete can also be recycled back to its original elements as well as into polyester fibres. Ethylene glycol is a colourless liquid. Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. To produce pet, chemists use two. How Is Pet Made Chemistry.
From www.politico.com
Petrochemical manufacturers use chemistry to make plastic more How Is Pet Made Chemistry Terephthalic acid and ethylene glycol. Pet is produced by the polymerization of ethylene glycol and terephthalic acid. Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. Polyethylene. How Is Pet Made Chemistry.
From www.hanlin.com
CIE A Level Chemistry复习笔记7.7.1 Formation of Polyesters翰林国际教育 How Is Pet Made Chemistry The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. Pet / pete can also be recycled back to its original elements as well as into polyester fibres. In order to synthesize pet the monomer of pet must be. Polyethylene terephthalate (pet) is a macromolecule called a polyester. To. How Is Pet Made Chemistry.
From essentialchemicalindustry.com
Polyesters How Is Pet Made Chemistry Pet is produced by the polymerization of ethylene glycol and terephthalic acid. Pet / pete can also be recycled back to its original elements as well as into polyester fibres. Terephthalic acid and ethylene glycol. Ethylene glycol is a colourless liquid. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one. How Is Pet Made Chemistry.
From www.mdpi.com
IJMS Free FullText An Overview into Polyethylene Terephthalate How Is Pet Made Chemistry Pet is produced by the polymerization of ethylene glycol and terephthalic acid. Ethylene glycol is a colourless liquid. Terephthalic acid and ethylene glycol. In order to synthesize pet the monomer of pet must be. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. To produce pet, chemists use. How Is Pet Made Chemistry.
From www.youtube.com
PET polymer or Polyethylene terephthalate preparation Industrially by How Is Pet Made Chemistry Pet / pete can also be recycled back to its original elements as well as into polyester fibres. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. In order to synthesize pet the monomer of pet must be. Both units undergo a. How Is Pet Made Chemistry.
From www.onlinemathlearning.com
Polymers IGCSE Chemistry (solutions, examples, worksheets, videos) How Is Pet Made Chemistry It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. In order to synthesize pet the monomer of pet must be. Terephthalic acid and ethylene glycol. Pet is produced by the polymerization of ethylene glycol and terephthalic acid. To produce pet, chemists use. How Is Pet Made Chemistry.
From www.alamy.com
PET, Polyethylene terephthalate, chemical formula and structure Stock How Is Pet Made Chemistry Polyethylene terephthalate (pet) is a macromolecule called a polyester. Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. Terephthalic acid and ethylene glycol. In order to synthesize pet the monomer of pet must be. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore,. How Is Pet Made Chemistry.
From stock.adobe.com
Structural chemical formula of polyethylene terephthalate molecule with How Is Pet Made Chemistry Polyethylene terephthalate (pet) is a macromolecule called a polyester. Terephthalic acid and ethylene glycol. Pet is produced by the polymerization of ethylene glycol and terephthalic acid. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. To produce pet, chemists use two different. How Is Pet Made Chemistry.
From pubs.rsc.org
Lowenergy catalytic methanolysis of How Is Pet Made Chemistry Polyethylene terephthalate (pet) is a macromolecule called a polyester. To produce pet, chemists use two different kinds of monomers: In order to synthesize pet the monomer of pet must be. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. It is a recyclable thermoplastic polymer with good strength,. How Is Pet Made Chemistry.
From chemistryfromscratch.org
V4.7 How Is Pet Made Chemistry Pet is produced by the polymerization of ethylene glycol and terephthalic acid. In order to synthesize pet the monomer of pet must be. Polyethylene terephthalate (pet) is a macromolecule called a polyester. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. To. How Is Pet Made Chemistry.
From www.tutormyself.com
4 Organic chemistry Archives TutorMyself Chemistry How Is Pet Made Chemistry Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. In order to synthesize pet the monomer of pet must be. Ethylene glycol is a colourless liquid. To produce pet, chemists use two different kinds of monomers: It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and. How Is Pet Made Chemistry.
From www.weprofab.com
PET Sheet Manufacturer and Supplier in China Weprofab How Is Pet Made Chemistry Polyethylene terephthalate (pet) is a macromolecule called a polyester. Terephthalic acid and ethylene glycol. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. To produce pet, chemists use two different kinds of monomers: Pet is produced by the polymerization of ethylene glycol. How Is Pet Made Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Polyethylene terephthalate How Is Pet Made Chemistry Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. Polyethylene terephthalate (pet) is a macromolecule called a polyester. Pet is produced by the polymerization of ethylene glycol and terephthalic acid. Ethylene glycol is a colourless liquid. In order to synthesize pet the monomer of pet must be. The. How Is Pet Made Chemistry.
From www.vectorstock.com
Polyethylene terephthalate pet pete polyester Vector Image How Is Pet Made Chemistry In order to synthesize pet the monomer of pet must be. Pet is produced by the polymerization of ethylene glycol and terephthalic acid. Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. To produce pet, chemists use two different kinds of monomers: Polyethylene terephthalate (pet) is a macromolecule. How Is Pet Made Chemistry.
From ar.inspiredpencil.com
Plastic Recycling Process Steps How Is Pet Made Chemistry Pet / pete can also be recycled back to its original elements as well as into polyester fibres. In order to synthesize pet the monomer of pet must be. Terephthalic acid and ethylene glycol. Ethylene glycol is a colourless liquid. Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and. How Is Pet Made Chemistry.
From surfguppy.com
Condensation Polymerization Surfguppy Chemistry made easy visual How Is Pet Made Chemistry Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. Pet is produced by the polymerization of ethylene glycol and terephthalic acid. Pet / pete can also be. How Is Pet Made Chemistry.
From www.researchgate.net
Structure of PET polymer. Polycondensation reaction of ethylene How Is Pet Made Chemistry Ethylene glycol is a colourless liquid. Pet is produced by the polymerization of ethylene glycol and terephthalic acid. The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection. How Is Pet Made Chemistry.
From www.mdpi.com
Polymers Free FullText BioPolyethylene (BioPE), Bio How Is Pet Made Chemistry Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. Ethylene glycol is a colourless liquid. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. To produce pet, chemists use two. How Is Pet Made Chemistry.
From www.vrogue.co
Flow Chart For Pet Bottles Recycling Washing Cleannin vrogue.co How Is Pet Made Chemistry Polyethylene terephthalate (pet) is a macromolecule called a polyester. Pet is produced by the polymerization of ethylene glycol and terephthalic acid. Pet / pete can also be recycled back to its original elements as well as into polyester fibres. To produce pet, chemists use two different kinds of monomers: The chemical structure of pet consists of repeating units of a. How Is Pet Made Chemistry.
From www.narodnatribuna.info
High Density Polyethylene Structure Properties And Uses How Is Pet Made Chemistry Pet is produced by the polymerization of ethylene glycol and terephthalic acid. Polyethylene terephthalate (pet) is a macromolecule called a polyester. Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. Pet / pete can also be recycled back to its original elements as well as into polyester fibres.. How Is Pet Made Chemistry.
From polymersinchemistry.blogspot.com
Polycondensation How Is Pet Made Chemistry The chemical structure of pet consists of repeating units of a group containing one ethylene glycol molecule and one terephthalic acid. Pet / pete can also be recycled back to its original elements as well as into polyester fibres. It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection. How Is Pet Made Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Copolymer How Is Pet Made Chemistry Both units undergo a reaction called esterification, where an organic acid and an alcohol combine to form an ester and water. To produce pet, chemists use two different kinds of monomers: It is a recyclable thermoplastic polymer with good strength, ductility, stiffness and hardness, therefore, can be processed through vacuum forming, injection moulding, compression moulding and blow moulding. Polyethylene terephthalate. How Is Pet Made Chemistry.