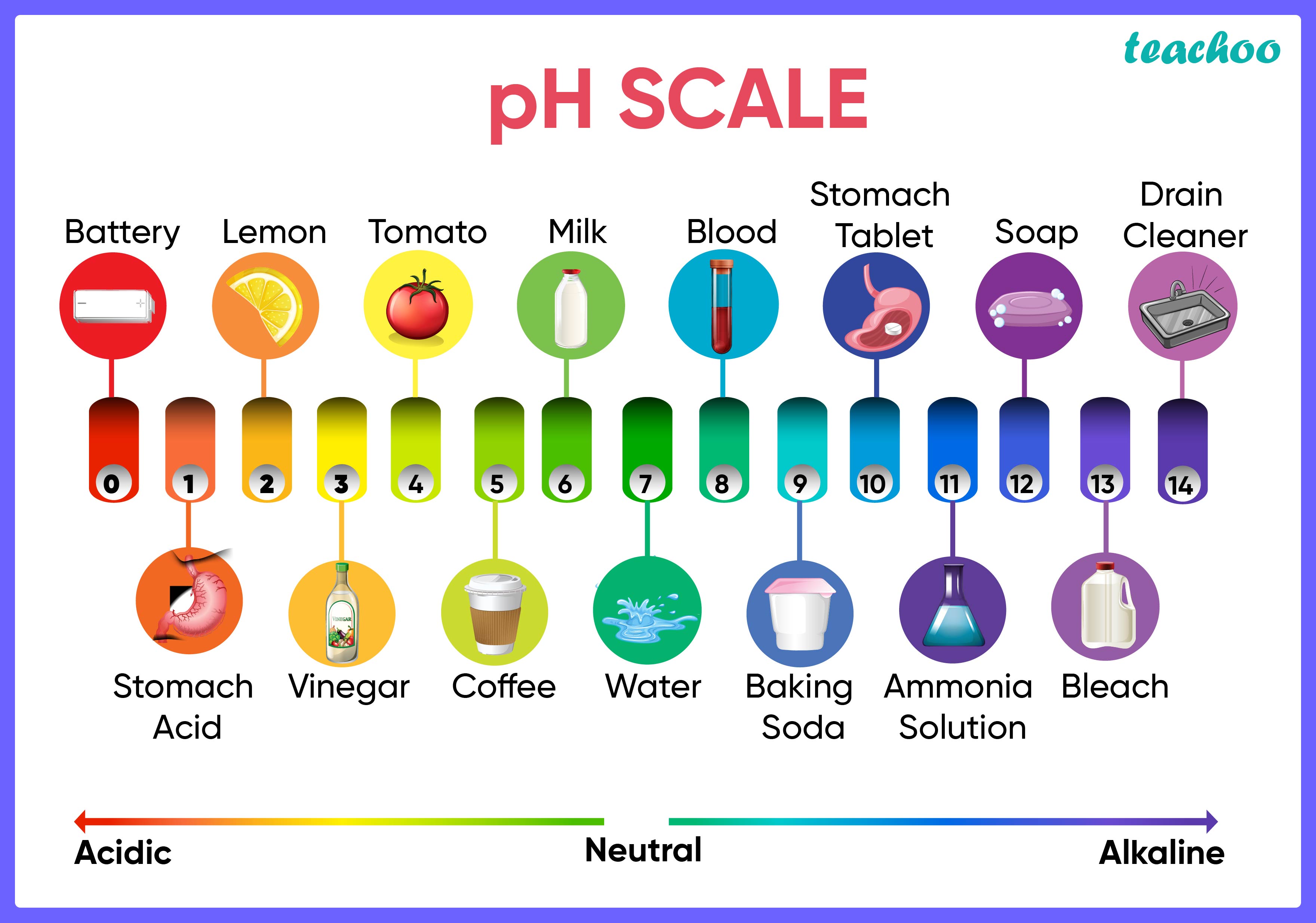
What Is Bases Science . a base is a substance that can neutralize the acid by reacting with hydrogen ions. bases are considered the chemical opposite of acids because of their ability to neutralize acids. the concepts of an acid, a base, and a salt are very old ones that have undergone several major refinements as. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. Aqueous solutions of bases are also. A is a metal oxide or metal hydroxide that neutralises an acid to produce a salt and water. scientists sometimes use another scheme — the lewis system — to define acids and bases. Bases have properties that mostly contrast with those of acids. Rbf, a widely used type of ann, is employed for regression and classification. In 1887 the swedish physicist and. The earliest definition of acids and bases is arrhenius's definition which states that: many cleaners contain ammonia, a base. Instead of protons, this lewis definition.
from www.teachoo.com
acids and bases are common classes of chemicals that react with each other and with water. Metal hydroxides, such as sodium hydroxide, or. Aqueous solutions of bases are also electrolytes. many cleaners contain ammonia, a base. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. Acidic substances are usually identified by their. Base is defined as the chemical compound whose ph value is greater than 7, accepts a proton,. the federal communications commission (fcc) has activated the disaster information reporting system (dirs) in response to. An alkali is a soluble. The weak bases have less ability to accept protons but the strong bases quickly accept protons in solution or from the donor molecules.
Strength of Acids and Bases (How to find it?) Chemistry Teachoo
What Is Bases Science bases are considered the chemical opposite of acids because of their ability to neutralize acids. many cleaners contain ammonia, a base. bases are considered the chemical opposite of acids because of their ability to neutralize acids. Base is defined as the chemical compound whose ph value is greater than 7, accepts a proton,. starcom to bring more than 450 military and civilian jobs to patrick space force base. a base is a molecule or ion able to accept a hydrogen ion from an acid. When it is soluble in water that known as alkali. arrhenius's definition of acids and bases. An acid is a substance. scientists sometimes use another scheme — the lewis system — to define acids and bases. Instead of protons, this lewis definition. Bases have properties that mostly contrast with those of acids. The weak bases have less ability to accept protons but the strong bases quickly accept protons in solution or from the donor molecules. Aqueous solutions of bases are also. the concepts of an acid, a base, and a salt are very old ones that have undergone several major refinements as. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media.
From www.slideserve.com
PPT Properties of Acids and Bases PowerPoint Presentation, free What Is Bases Science The base has the ability to accept a proton from a donor. In 1887 the swedish physicist and. Bases have properties that mostly contrast with those of acids. When it is soluble in water that known as alkali. The weak bases have less ability to accept protons but the strong bases quickly accept protons in solution or from the donor. What Is Bases Science.
From examples.yourdictionary.com
Difference Between Acids and Bases Key Properties What Is Bases Science The earliest definition of acids and bases is arrhenius's definition which states that: Rbf, a widely used type of ann, is employed for regression and classification. bases are considered the chemical opposite of acids because of their ability to neutralize acids. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions. What Is Bases Science.
From www.chemicals.co.uk
What Is a Base in Chemistry? The Chemistry Blog What Is Bases Science Aqueous solutions of bases are also. many cleaners contain ammonia, a base. a base is a substance that can neutralize the acid by reacting with hydrogen ions. radial basis function (rbf) neural network. The base has the ability to accept a proton from a donor. When it is soluble in water that known as alkali. Metal hydroxides,. What Is Bases Science.
From www.youtube.com
BASES CBSE NCERT Science YouTube What Is Bases Science the federal communications commission (fcc) has activated the disaster information reporting system (dirs) in response to. Sodium hydroxide is found in drain cleaner. a base is a molecule or ion able to accept a hydrogen ion from an acid. Aqueous solutions of bases are also electrolytes. many cleaners contain ammonia, a base. The weak bases have less. What Is Bases Science.
From kidspressmagazine.com
Acids and Bases What Is Bases Science a base is a substance that neutralises acids. Instead of protons, this lewis definition. base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the. Most bases are minerals that react with acids. Aqueous solutions of bases are also electrolytes. starcom to bring more than 450 military and civilian jobs. What Is Bases Science.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2789112 What Is Bases Science starcom to bring more than 450 military and civilian jobs to patrick space force base. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. the concepts of an acid, a base, and a salt are very old ones that have undergone several major refinements. What Is Bases Science.
From www.reliableeducationgroups.in
Acids, Bases and Salts Class 7 Science Notes Chapter 5 Reliable What Is Bases Science When it is soluble in water that known as alkali. Sodium hydroxide is found in drain cleaner. A is a metal oxide or metal hydroxide that neutralises an acid to produce a salt and water. a base is a molecule or ion able to accept a hydrogen ion from an acid. Instead of protons, this lewis definition. a. What Is Bases Science.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo What Is Bases Science many cleaners contain ammonia, a base. a base is a substance that neutralises acids. Rbf, a widely used type of ann, is employed for regression and classification. base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the. Metal hydroxides, such as sodium hydroxide, or. arrhenius's definition of acids. What Is Bases Science.
From exokdcsll.blob.core.windows.net
Examples Of Household Acids And Bases at Pennington blog What Is Bases Science Aqueous solutions of bases are also. An alkali is a soluble. arrhenius's definition of acids and bases. Bases have properties that mostly contrast with those of acids. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. A is a metal oxide or metal hydroxide that. What Is Bases Science.
From www.youtube.com
Acids, Bases and Salts Class 10 Full Chapter (Animation) Class 10 What Is Bases Science As a result, they are important. Metal oxides, such as copper oxide. Most bases are minerals that react with acids. radial basis function (rbf) neural network. The earliest definition of acids and bases is arrhenius's definition which states that: starcom to bring more than 450 military and civilian jobs to patrick space force base. bases are considered. What Is Bases Science.
From studylib.net
ACIDS AND BASES What Is Bases Science radial basis function (rbf) neural network. The weak bases have less ability to accept protons but the strong bases quickly accept protons in solution or from the donor molecules. acids and bases are common classes of chemicals that react with each other and with water. In 1887 the swedish physicist and. The earliest definition of acids and bases. What Is Bases Science.
From www.tffn.net
What is Base Science? An InDepth Exploration of Its Definition and What Is Bases Science the federal communications commission (fcc) has activated the disaster information reporting system (dirs) in response to. radial basis function (rbf) neural network. a base is a molecule or ion able to accept a hydrogen ion from an acid. a base is a substance that can neutralize the acid by reacting with hydrogen ions. a base. What Is Bases Science.
From dxofrjqld.blob.core.windows.net
What Is Acids And Bases Examples at Tyler Warner blog What Is Bases Science a base is a molecule or ion able to accept a hydrogen ion from an acid. bases are considered the chemical opposite of acids because of their ability to neutralize acids. starcom to bring more than 450 military and civilian jobs to patrick space force base. Rbf, a widely used type of ann, is employed for regression. What Is Bases Science.
From ar.inspiredpencil.com
Base Science Definition What Is Bases Science Rbf, a widely used type of ann, is employed for regression and classification. Aqueous solutions of bases are also. Most bases are minerals that react with acids. Base is defined as the chemical compound whose ph value is greater than 7, accepts a proton,. acids and bases are common classes of chemicals that react with each other and with. What Is Bases Science.
From ar.inspiredpencil.com
Base Science What Is Bases Science a base is a molecule or ion able to accept a hydrogen ion from an acid. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also. arrhenius's definition of acids and bases. bases are considered the chemical opposite of acids because of their ability to neutralize acids. When it is soluble. What Is Bases Science.
From exokqjepn.blob.core.windows.net
Bases Definition English at Phyllis Miller blog What Is Bases Science Bases have properties that mostly contrast with those of acids. a base is a substance that can neutralize the acid by reacting with hydrogen ions. Acidic substances are usually identified by their. Most bases are minerals that react with acids. Metal oxides, such as copper oxide. Instead of protons, this lewis definition. The weak bases have less ability to. What Is Bases Science.
From www.worksheetsplanet.com
What is a Base Definition of Base What Is Bases Science When it is soluble in water that known as alkali. arrhenius's definition of acids and bases. a base is a molecule or ion able to accept a hydrogen ion from an acid. Aqueous solutions of bases are also. Metal oxides, such as copper oxide. in the field of chemistry, a ‘base’ can be defined as a substance. What Is Bases Science.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo What Is Bases Science Metal hydroxides, such as sodium hydroxide, or. acids and bases are common classes of chemicals that react with each other and with water. An acid is a substance. Metal oxides, such as copper oxide. radial basis function (rbf) neural network. arrhenius's definition of acids and bases. base, in chemistry, any substance that in water solution is. What Is Bases Science.
From www.slideserve.com
PPT Chemical properties and changes of matter PowerPoint Presentation What Is Bases Science The earliest definition of acids and bases is arrhenius's definition which states that: radial basis function (rbf) neural network. The base has the ability to accept a proton from a donor. Metal oxides, such as copper oxide. scientists sometimes use another scheme — the lewis system — to define acids and bases. base, in chemistry, any substance. What Is Bases Science.
From www.slideserve.com
PPT Acids & Bases What’s the difference??? PowerPoint Presentation What Is Bases Science Metal hydroxides, such as sodium hydroxide, or. acids and bases are common classes of chemicals that react with each other and with water. When it is soluble in water that known as alkali. An alkali is a soluble. The earliest definition of acids and bases is arrhenius's definition which states that: base, in chemistry, any substance that in. What Is Bases Science.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID9013474 What Is Bases Science Most bases are minerals that react with acids. many cleaners contain ammonia, a base. Bases have properties that mostly contrast with those of acids. scientists sometimes use another scheme — the lewis system — to define acids and bases. Aqueous solutions of bases are also. An acid is a substance. Instead of protons, this lewis definition. the. What Is Bases Science.
From www.tffn.net
What is Base Science? An InDepth Exploration of Its Definition and What Is Bases Science Instead of protons, this lewis definition. many cleaners contain ammonia, a base. the federal communications commission (fcc) has activated the disaster information reporting system (dirs) in response to. A is a metal oxide or metal hydroxide that neutralises an acid to produce a salt and water. Metal hydroxides, such as sodium hydroxide, or. a base is a. What Is Bases Science.
From learningraminasi25.z14.web.core.windows.net
How To Identify Acids And Bases In A Equation What Is Bases Science Aqueous solutions of bases are also electrolytes. arrhenius's definition of acids and bases. The base has the ability to accept a proton from a donor. scientists sometimes use another scheme — the lewis system — to define acids and bases. Metal oxides, such as copper oxide. in the field of chemistry, a ‘base’ can be defined as. What Is Bases Science.
From www.docsity.com
Science Acids Bases and Salts Complete Handwritten Notes Docsity What Is Bases Science The earliest definition of acids and bases is arrhenius's definition which states that: Most bases are minerals that react with acids. Bases have properties that mostly contrast with those of acids. Acidic substances are usually identified by their. In 1887 the swedish physicist and. The base has the ability to accept a proton from a donor. Base is defined as. What Is Bases Science.
From www.teachoo.com
Uses of Base 5+ Examples Chemistry Teachoo Teachoo Questions What Is Bases Science Bases have properties that mostly contrast with those of acids. The base has the ability to accept a proton from a donor. As a result, they are important. arrhenius's definition of acids and bases. base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the. scientists sometimes use another scheme. What Is Bases Science.
From www.teachoo.com
Bases and it's Properties (with Examples, Definition) Teachoo What Is Bases Science starcom to bring more than 450 military and civilian jobs to patrick space force base. Metal oxides, such as copper oxide. In 1887 the swedish physicist and. scientists sometimes use another scheme — the lewis system — to define acids and bases. Base is defined as the chemical compound whose ph value is greater than 7, accepts a. What Is Bases Science.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free What Is Bases Science Rbf, a widely used type of ann, is employed for regression and classification. many cleaners contain ammonia, a base. Acidic substances are usually identified by their. bases are considered the chemical opposite of acids because of their ability to neutralize acids. acids and bases are common classes of chemicals that react with each other and with water.. What Is Bases Science.
From www.slideserve.com
PPT Chapter 23 Acids, Bases, and Salts PowerPoint Presentation, free What Is Bases Science Aqueous solutions of bases are also electrolytes. An acid is a substance. Metal hydroxides, such as sodium hydroxide, or. the federal communications commission (fcc) has activated the disaster information reporting system (dirs) in response to. A is a metal oxide or metal hydroxide that neutralises an acid to produce a salt and water. Acidic substances are usually identified by. What Is Bases Science.
From www.geeksforgeeks.org
What are Bases? Definition, Examples, Types, Properties and Uses What Is Bases Science A is a metal oxide or metal hydroxide that neutralises an acid to produce a salt and water. Sodium hydroxide is found in drain cleaner. Bases have properties that mostly contrast with those of acids. Metal oxides, such as copper oxide. the federal communications commission (fcc) has activated the disaster information reporting system (dirs) in response to. Aqueous solutions. What Is Bases Science.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry What Is Bases Science scientists sometimes use another scheme — the lewis system — to define acids and bases. arrhenius's definition of acids and bases. The earliest definition of acids and bases is arrhenius's definition which states that: Rbf, a widely used type of ann, is employed for regression and classification. Metal oxides, such as copper oxide. Bases have properties that mostly. What Is Bases Science.
From sciencenotes.org
Facts About Acids and Bases What Is Bases Science arrhenius's definition of acids and bases. Aqueous solutions of bases are also. a base is a molecule or ion able to accept a hydrogen ion from an acid. Bases have properties that mostly contrast with those of acids. Most bases are minerals that react with acids. Bases have properties that mostly contrast with those of acids. The earliest. What Is Bases Science.
From www.slideserve.com
PPT Chapter 23 Acids, Bases, and Salts PowerPoint Presentation, free What Is Bases Science acids and bases are common classes of chemicals that react with each other and with water. An acid is a substance. In 1887 the swedish physicist and. Rbf, a widely used type of ann, is employed for regression and classification. Metal hydroxides, such as sodium hydroxide, or. The weak bases have less ability to accept protons but the strong. What Is Bases Science.
From ar.inspiredpencil.com
Base Science What Is Bases Science radial basis function (rbf) neural network. The weak bases have less ability to accept protons but the strong bases quickly accept protons in solution or from the donor molecules. many cleaners contain ammonia, a base. a base is a molecule or ion able to accept a hydrogen ion from an acid. in the field of chemistry,. What Is Bases Science.
From ar.inspiredpencil.com
Base Science What Is Bases Science a base is a substance that can neutralize the acid by reacting with hydrogen ions. base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the. scientists sometimes use another scheme — the lewis system — to define acids and bases. a base is a molecule or ion able. What Is Bases Science.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs What Is Bases Science The base has the ability to accept a proton from a donor. Bases have properties that mostly contrast with those of acids. acids and bases are common classes of chemicals that react with each other and with water. many cleaners contain ammonia, a base. An alkali is a soluble. the concepts of an acid, a base, and. What Is Bases Science.